As clinical trials continue to grow globally, technology plays an important role in expediting the study start-up and the maintenance of data integrity. Clinical trials in Asia have witnessed an average yearly growth of 30% from 2005 to 20121. If a study management team has to undertake international travel to conduct investigator meetings, travel alone will take up a large portion of the team’s time and energy.
Therefore, study teams are seeking the help of technology to reduce this effort in all aspects of clinical trials: from preclinical research and development and regulatory communication to packaging and distribution and post-marketing studies.
Site personnel qualification technology can be made effective if it is targeted to each role in a clinical research site. If this is not done, individuals receive too much material or inappropriate material concerning their function within the study.
UL Compliance to Performance offers clinical site qualification technologies to the life sciences sector. The company has formulated four guiding rules to structure an automated clinical qualification solution, within a company’s clinical system architecture, in order to provide the following benefits:
- Enhance the role-based performance of clinical site personnel
- Speed up the ramp-up time for new clinical sites
- Improve the performance and wisdom of investigators
- Decrease the time associated with audit preparation during regulatory audits
- Display commitment to well-trained staff at the time of sponsor audits
- Assure compliance with protocol and non-protocol needs
- Decrease the total site personnel qualification costs
Study teams can show an international commitment to compliance and quality by employing technology to manage site personnel training and employee training.
Key topics
This article focuses on the following three topics:
- eClinical’s Role in Improving Employee and Site Performance
- Defining the Goals of an eClinical Qualification Solution
- Four Guiding Rules for Structuring an eClinical Training Solution
eClinical’s role in clinical site performance
Clinical trials potentially denote the costliest and most time-consuming phase of product development. A study can involve multiple sites and hundreds of patients, while sponsors face challenges to speed up site initiation.
Conventionally, an in-person approach was employed to train site personnel and employees on GCP fundamentals, study protocols, and even electronic data capture (EDC) technology. However, this strategy will not be cost-effective if sites and employees become more dispersed. The strategy also results in a heavy administrative burden in “capturing” qualification and training records, for the purpose of audits.
Sponsors looking for an eClinical qualification solution should think in terms of online forms, eLearning, integration tools, and the ability to capture instructor-led training, such as investigator meetings. For instance, UL clients trust ComplianceWire®, being a secure web-based system, it enables CROs and sponsors to add eClinical training for clinical research sites and employees:
- “Read and understood” records related to site personnel must be distributed and captured for all protocol-related materials
- SOPs, online GCP training courses, and other non-protocol items must also be managed
On average, clients have reported as much as 40% or more reduction in site initiation training expenditure after eClinical Qualification programs have been included in the clinical ecosystem (CTMS, EDC, etc.). Clients have also reported that site personnel and employees have displayed enhanced proficiency within studies.
The eClinical qualification solution should have the capability to cut down noncompliance risks. While the costs of patient and investigator recruitment on clinical trials have attracted more attention, noncompliance with regulatory requirements denotes one of the most substantial as well as unnecessary risks to a clinical study’s research integrity and profits.
Sketchy knowledge among clinical investigators and support staff, corporate compliance personnel, and investigational review boards, can lead to delayed clinical studies and postpone product approval by regulatory authorities.
eClinical documents compliant with clinical research materials allow service providers and CROs to evaluate effectiveness using real data, instead of relying on only in-person training observations.
Defining the goals of an eClinical qualification solution
Irrespective of a study’s location, the sale of medical products in the US market needs the approval of the FDA for the study results and compliance with FDA-specified standards of knowledge, critical information management and documentation.
Study teams have the following compliance and business goals:
- Provide consistent and timely updates of important study information to external study teams and project teams
- Obtain assurances that the study and product information has been received and understood by the responsible person
- Begin or complete post-marketing commitments according to schedule
It is possible that these goals may differ across the clinical industry; however, it is important that the eClinical qualification solution achieves the company’s goals and demonstrates value to employees as well as site personnel.
The eClinical qualification solution should also show a strong return on investment (ROI) to senior managers in finance and quality departments. To track eClinical training requirements that allow organizations to reach these objectives, companies begin with the following seven criteria:
Web-based technology
A Software as a Service solution (SaaS) enables a cost-efficient “pay as you go” pricing model, convenient “anywhere” access for employees and third parties, simultaneous global distribution, and reduced IT investment.
High security
The system must include a validated and secure infrastructure that allows electronic communication with study personnel, surpassing the challenge of opening up a sponsor’s internal firewall to many external parties; for the purposes of data integrity, the system should satisfy FDA’s rigorous computer validation requirements.
Ability to host content
Instead of a clinical researcher or site manager reviewing documents located on an internal network server, sponsors can store important protocols, amendments, conflicts of interest forms, patient recruitment criteria, and other documents on a secure server, thus lowering multiple network sign-ins. Thus, the user experience is enhanced by a secure content hosting option while avoiding the strain on the company’s IT support team.
Integrate with key applications
The system should be able to easily integrate with the current clinical ecosystem, which may include the document management system, clinical trial management system, or EDC in order to enable a seamless process that directly presents a researcher’s qualification on the CTMS.
After integration is achieved, site personnel are automatically added to eClinical training system, and new qualification assignments are automatically triggered by protocol amendments.
“Testing out” of training
Experienced employees and personnel should be able to “test out” of specific training content such as core GCP principles, through the eClinical qualification solution, particularly if training is a yearly necessity. For instance, UL builds courses that allow site personnel to “test out” of specific topics, according to an automated “pre-test” covering the entire course.
Deliver flexible reporting
The sponsor company, by using the eClinical qualification solution, should be able to know the training status of investigators and other site personnel for future recruitment.
Ability to grant third-party credits
The site managers, by using the eClinical qualification solution, should be able to “grant” credit to GCP educational activities carried out by site personnel, thus enabling individuals to upload certificates from the completed GCP education activities.
About UL’s eClinical qualification solution
UL’s ComplianceWire® is used by many companies to manage training for all employees as well as study project managers, research coordinators, investigators, and monitors. UL’s Clinical Libraries cover topics such as the Good Guidance Practice (GGP), FDA’s Bioresearch Monitoring (BIMO) program, HIPAA, informed consent and specific roles within a study.
All training can be organized and tracked for audit purposes. Additionally, the UL Clinical Learning Platform provides assessment tools to allow sponsors to get a confirmation on the study personnel’s interpretation of key study knowledge and obligations.
By partnering with the FDA’s Office of Regulatory Affairs (ORA), UL has delivered online training, documentation tracking, and technology designed to satisfy 21 CFR Part 11 requirements for the FDA’s virtual university, ORA-U.
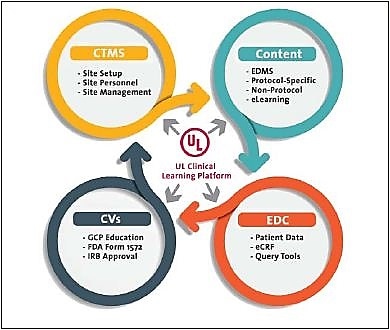
Four guiding rules for embedding eClinical qualification
After satisfying the requirements, the organization’s next logical step is to structure the eClinical qualification solution, achieving the stated compliance and business metrics.
As stated earlier, usually these objectives focus on satisfying regulatory obligations, streamlining the approval process, improving safety, strengthening site personnel’s competencies, standardizing training qualifications, and reducing administrative time and effort.
Based on the experience with UL’s own compliance training platform, the company recommends four rules for building eClinical qualification framework:
Aligning training requirements with clinical roles
Timely, accurate distribution of protocols, GCP content and other areas (such as use of EDC applications) to all responsible parties are required to ensure training compliance.
Consistent naming conventions are required to ensure that the administrator has the required ability to identify the right document for reporting and modification purposes. The eClinical Learning Management System (LMS) must provide these features:
- Organizing each study by sponsor, geography, product, etc.
- Building User Groups by title or role (PI, CRC, CRA, etc.)
- Identifying qualification criteria and activities that constitute the criteria. A chart demonstrating a typical Clinical Training and Reporting Structure is given below. The categories should be divided into protocol and non-protocol activities (for example, GCP requirements). The activities need to be structured into curricula.
|
Clinical education structure
|
|
Category
|
Examples
|
|
GCP training
|
US, Europe, Asia
|
|
Role-specific
|
Primary investigator
Sub-investigator
Monitor
Study coordinator
|
|
Study-specific
|
Protocol, reports
|
For instance, the site manager may need courses highlighting the internal legal responsibilities of monitors, sponsors, and investigators; investigational site selection; monitoring requirements; selection of investigators; and the role and responsibilities of Institutional Review Boards (IRBs).
However, other than these essentials, additional courses of study may be required ranging from understanding international ethics requirements to international standards of informed consent.
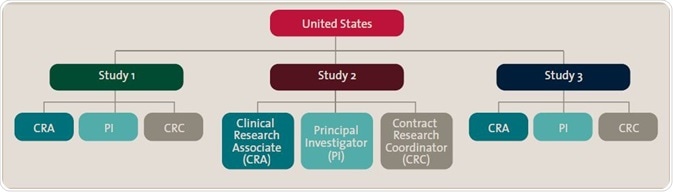
The chart illustrates qualification roles structured first by country, then specific study, then roles within that study. This enables specific content to be distributed once to all individuals according to the organization’s particular structure.
Building a training hierarchy that accommodates growth
In several cases, the system may expand as the CRO gains more sponsors and manages trials globally. Thus, it is important that each clinical research associate and investigator within each study, and in each location, receive only the appropriate training concerning their role.
For this purpose, the eClinical qualification solution needs to be flexible to accommodate the client’s unique training structure. Within ComplianceWire®, clients gain several efficiencies of grouping site personnel in this type of hierarchy:
- Reducing training content overlap
- Targeted reporting to identify training gaps
- Automatic maintenance of training assignments when new site personnel are added to specific user groups
These qualification standards allow meaningful and consistent reporting metrics. The metrics are measurable at all levels of the organization down to specific roles within the clinical study.
The roles within each study can be divided, so that the system administrator or site manager can conveniently maintain the content for a specific role within a specific study, and produce qualification reports in order to identify knowledge gaps.
Developing a process for maintaining site personnel data
The clinical management team should define and store worthwhile user data features in order to organize and align site personnel into defined training requirements. This can be achieved through automated or manual means.
The ability to view real-time information and data integrity are critical. Common “user” features, such as name, organization, and e-mail, can be fed from the CTMS. Additionally, the eClinical qualification solution must employ four clinical site personnel “features”: site, study, role, and region, so as to offer segmented visibility to the sponsor’s site managers.
Provide segmented management visibility
The delivery and tracking of qualifications need to be divided, so that study managers can only see the progress of site personnel as it is associated with the features stated above. Ideally, these attributes must be exhibited at the location in which site personnel records are stored.
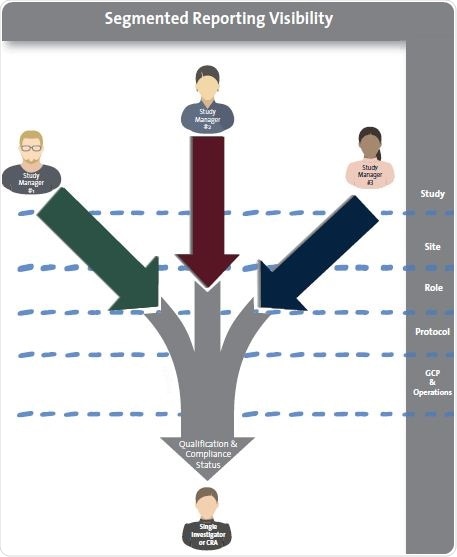
Successful site personnel qualification requires that study, site, role and protocol attributes be attached to each individual in the study. This ensures that each study manager only gains visibility into the specific qualification of that individual.
Acknowledgements
Produced from materials originally authored by Scott Barnard, Marketing Strategy Director, UL Compliance to Performance.
References
- Globalization of clinical trials: ethical and regulatory implications, International Journal of Clinical Trials , da Silva RE et al. Int J Clin Trials. 2016 Feb;3(1):1-8
About UL Compliance to Performance
UL Compliance to Performance provides knowledge and expertise that empowers Life Sciences organizations globally to accelerate growth and move from compliance to performance.
Our solutions help companies enter new markets, manage compliance, optimize quality and elevate performance by supporting processes at every stage of a company’s evolution.
UL provides a powerful combination of advisory solutions with a strong modular SaaS backbone that features ComplianceWire®, our award-winning learning and performance platform.
UL is a premier global independent safety science company that has championed progress for 120 years. It’s more than 12,000 professionals are guided by the UL mission to promote safe working and living environments for all people.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.