Bromodomain protein 4 (BRD4), a transcriptional regulator, plays a crucial role in autoimmunity, cancer, and inflammatory diseases.1,2 Researchers found that BRD4 is a protein attached to acetylated chromatin at the time of cell cycle progression.
Thus, BRD4 keeps gene expression consistent during further rounds of division, a phenomenon called epigenetic memory or 'bookmarking' for gene transcription.2,7 In addition, BRD4 has a vital role in the regulation of cell differentiation and development.2,4
The lack of BRD4 inhibits the ability of the bone marrow stem cells to produce B and T cells.2 BRD4 is essential for the re-expression of stem cell genes while reprogramming B cells or MEFs to induced pluripotent stem cells.2 It also has a role in osteoblast differentiation.4
Thanks to its role in cell cycle control and differentiation, BRD4 has become an emerging therapeutic target for immune system and cancer pathologies.
.jpg)
Figure 1. Diagram of full length human BRD4 showing its four primary functions: (1) binding to acetylated chromatin via two N terminal bromodomains (BD1, BD2), (2) histone acetyl transferase (HAT) activity for acetylating lysine residues in histones, (3) serine kinase activity which acts on RNA pol II, and (4) a C-terminal motif (CTM) which serves as a nucleation site for transcription factors. The extraterminal (ET) domain is characteristic of BET family members and serves as an additional protein-protein interaction domain. Image Credit: Bethyl Laboratories Inc.
Molecular functions of BRD4 protein
In the BET class of bromodomain proteins, two bromodomains and an ET domain are present.2,6 Among these proteins, BRD4 is the most well-examined and has four main molecular functions:
- It maintains chromatin structure through the binding of acetylated histones, which is an activity induced by sites in the N-terminal domain.
- It carries out Histone Acetyl Transferase (HAT) activity through its HAT catalytic domain.2,6
- It supports serine kinase activity through a kinase domain that spans the phosphorylates residues and N-terminal region in the C-terminal domain of RNA Pol II.2,6
- The C-terminal domain acts as a nucleation and binding site for transcription factors and complexes.
BRD4 occurs in various transcription complexes, such as the general cofactor Mediator and the P-TEFb elongation factor.6 The C terminal domain induces the interactions of BRD4 with several familiar transcriptional regulators, most importantly MYC, P-TEFb, p53 and NFκb.2,4,6–8
Recently, BRD4 has emerged as a potential target for cancer therapy due to its role as an epigenetic and transcriptional regulator of the cell cycle.1–5 Specifically, several hematopoietic cancers rely on continuous BRD4 activity for Myc expression.2,3,5,7 Even solid tumors are related to BRD4 activity.2
BRD4 deregulation is clinically associated with colon, breast and prostate cancers.7 Fascinatingly, BRD4-NUT - a BRD4 fusion protein - occurs in aggressive midline carcinomas.2,7 However, it is not clear whether BRD4 has an additional role in metastasis.2
Until now, most available BET inhibitors influence all members of the BET family that produce unpredictable and at times dangerous results.1
BET inhibitors block the link between BRD4 and chromatin by simulating acetyl-lysine residues on histones.2,3 This impact avoids transcription of Myc as well as other crucial regulatory genes resulting in cell cycle arrest.2,3,5
Certain BET inhibitors are now being developed, which will enable more selective suppression of BRD4 and other members of the BET family.1,3,5,7
BET inhibitors might be specifically effective when used together with a range of other chemotherapeutic agents by facilitating the use of lower doses of toxic drugs and by supporting to overcome resistance.5,7
Bethyl recognizes the importance of BRD4 in tumorigenesis and cell cycle regulation, and thus manufactures rigorously tested antibodies to BRD4 and several other associated proteins to support cancer research advancement.
Associated products
Recommended secondaries
- Rabbit IgG-heavy and light chain Antibody (A120-101P)
- Rabbit IgG-heavy and light chain cross-adsorbed Antibody (A120-201D3)
- Rabbit IgG-heavy and light chain cross-adsorbed Antibody (A120-201D4)
- Goat anti-Rabbit IgG-heavy and light chain cross-adsorbed Antibody (A120-201D5)
- Rabbit IgG-heavy and light chain highly cross-adsorbed Antibody (A120-501P)
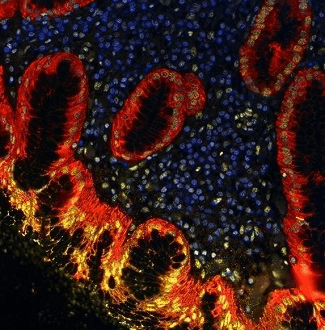
Figure 2. Detection of human BRD4 (yellow) and Cytokeratin (red) in FFPE gastric carcinoma by IHC-IF. Antibody: Rabbit anti-BRD4 recombinant monoclonal [BL-149-2H5] (A700-004) and mouse anti-Cytokeratin monoclonal [AE1/AE3] (A500-019A). Secondary: DyLight® 650-conjugated goat anti-rabbit IgG (A120-201D5). Image Credit: Bethyl Laboratories Inc.
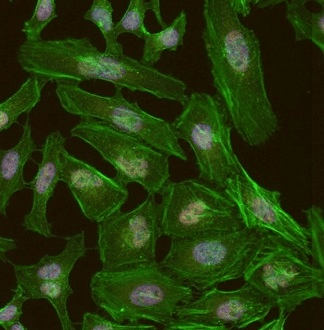
Figure 3. Detection of human BRD4 in formaldehyde-fixed HeLa cells by ICC-IF. Antibody: Rabbit anti-BRD4 recombinant monoclonal [BL-149-2H5] (A700-004). Secondary: DyLight® 550-conjugated goat anti-rabbit IgG cross-adsorbed (A120-201D3). Counterstain: Phalloidin Alexa Fluor® 488. Image Credit: Bethyl Laboratories Inc.
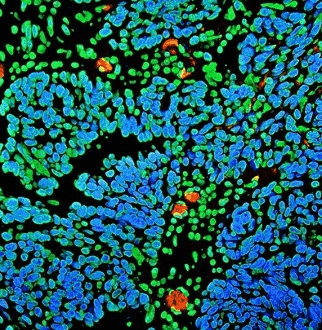
Figure 4. Detection of human BRD4 in FFPE ovarian carcinoma by IHC-IF (pseudo color). Antibody: Rabbit anti-BRD4 recombinant monoclonal [BL-149-2H5] (A700-004). Secondary: DyLight® 594-conjugated goat anti-rabbit IgG cross-adsorbed (A120-201D4). Counterstain: DAPI. Image Credit: Bethyl Laboratories Inc.
References
- Andrieu G, Belkina AC, Denis GV. 2016. Clinical trials for BET inhibitors run ahead of the science. Drug Discov Today Technol. Mar;19:45–50.
- Devaiah BN, Gegonne A, Singer DS. 2016. Bromodomain 4: a cellular Swiss army knife. J Leukoc Biol. Oct;100(4):679–686.
- Liu Z, Wang P, Chen H, Wold EA, Tian B, Brasier AR, Zhou J. 2017. Drug Discovery Targeting Bromodomain-Containing Protein 4. J Med Chem. Jun 8;60(11):4533–4558.
- Najafova Z, Tirado-Magallanes R, Subramaniam M, Hossan T, Schmidt G, Nagarajan S, Baumgart SJ, Mishra VK, Bedi U, Hesse E, Knapp S, Hawse JR, Johnsen SA. 2016. BRD4 localization to lineage-specific enhancers is associated with a distinct transcription factor repertoire. Nucleic Acids Res. Jan 9;45(1):127–141.
- Ocaña A, Nieto-Jiménez C, Pandiella A. 2017. BET inhibitors as novel therapeutic agents in breast cancer. Oncotarget Aug 1;8(41):71285–71291.
- Wu SY, Chiang CM. 2007. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J Biol Chem. May 4;282(18):13141–13145.
- Wu SY, Lee AY, Lai HT, Zhang H, Chiang CM. 2013. Phospho switch triggers Brd4 chromatin binding and activator recruitment for gene-specific targeting. Mol Cell. Mar 7;49(5):843–857.
- Yang Z, He N, Zhou Q. 2008. Brd4 Recruits P-TEFb to Chromosomes at Late Mitosis To Promote G1 Gene Expression and Cell Cycle Progression. Mol Cell Biol. Feb;28(3):967–976.
About Bethyl Laboratories, Inc.

Bethyl Laboratories, Inc. has been dedicated to improving lives by supporting scientific discovery through its qualified antibody products and custom polyclonal services since its founding in 1972. Bethyl has a global reputation for quality, consistency and first-class customer care. Every antibody that Bethyl sells is manufactured to exacting standards in Montgomery, Texas, and is validated in-house by a team of scientists. From the veterinary facilities to the development, production, and validation labs, the entire Bethyl team focuses on delivering quality products and delighting customers.
Bethyl Laboratories has been acquired by Fortis Life Sciences. To learn more visit: https://www.fortislife.com/fortis-life-sciences-acquires-bethyl-laboratories.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.