Vaccines are one of the most critical medical advancements in human history. This was recently demonstrated with the international coronavirus pandemic.
The highly transmissible COVID-19 virus resulted in lockdowns being placed in many countries around the globe, during which only companies offering necessary services and supplies were allowed to remain open, and the general public, with the exception of key workers, were legally required to stay indoors.
The production of vaccines against this virus allowed these restrictions to be safely lifted by decreasing transmission of the virus and helping contagious individuals to rid the virus from the body more efficiently, hindering the intensity of symptoms.
Since the rabies vaccine was first created by Louis Pasteur, vaccines have been employed to optimize the immune systems of total populations against life-threatening and debilitating diseases, such as diptheria, polio, smallpox and measles.
This has evidently been a highly successful approach, where the proliferation of many diseases has been controlled. Systematic vaccination eliminated smallpox that had historically been the cause of 1 in every 13 deaths.
It has been difficult to produce vaccinations against diseases that are caused by pathogens that enter cells. This is due to the fact that antibodies created from vaccination (or infection) travel through the bloodstream but do not infiltrate cells.
A significant T cell response is required to eliminate the infected cells, and this is normally not possible with the contemporary live attenuated vaccines, for example, BCG that is utilized against intracellular pathogens.
The immune response of T cells is informed by dendritic cells, which means these cells are now the main focus of new vaccine designs.
Lentiviral vectors, in particular, are being employed in the development of vaccines that target dendritic cells, facilitating a significant T cell immune response. In turn, this allows intracellular pathogens such as malaria, tuberculosis and HIV to be removed.
Lentiviral vectors
Viral vectors continue to be the fastest method of transporting genetic material into host cells. According to the virus used, various kinds of genetic material can be inserted, including both double- and single-stranded RNA or DNA.1
A number of distinct virus types have been employed, with the most popular being retroviruses, adenoviruses and adeno-associated viruses. Lentiviruses are now being selected more often due to their ability to predominantly infect dendritic cells.
Nondividing cells can be transduced by lentiviruses as they can translocate throughout the nuclear membrane. They are connected to a low risk of insertional mutagenesis when the target gene is included due to their distinct patterns of integration.
With lentiviral vectors, the challenge of insertional mutagenesis continues to be a concern which means there is still a need for new vector development.
There are also non-integrative lentiviral vectors that do not insert their genetic material into that of the host. This reduces the chance of failure or undesirable additional effects.
Lentiviruses more crucially offer a sustained demonstration of antigens by dendritic cells, facilitating a fast T cell response, which is advantageous for both disease prevention through vaccination and in curing disease, for example, in cancer therapy.
Applications of lentiviral vectors
Lentiviral vectors can be utilized in medicine for both therapeutic applications, including in gene therapy and cancer therapy and in prophylactic uses, such as in vaccines.
Many clinical and animal studies have shown the effectiveness of lentiviral vectors for a broad range of applications.
Prophylactic applications
Vaccines that utilize a lentiviral vector have been proven to trigger a significant immune response against a range of pathogens.
Administering non-integrative lentiviral vector-based vaccines to mice models offered long-term defense against malaria,2 tuberculosis3 and the Zika virus.4
Similarly, using a lentiviral vector vaccine in the immunization of pigs elicited long-lasting immunity to the Japanese encephalitis virus, the main driver of viral encephalitis in Southeast Asia.5
A vaccine against the SARS-CoV-2 (COVID-19) virus has recently been produced utilizing a lentiviral vector.6,7 Interestingly, in hamster and mice models, the COVID-19 vaccine was successful after intranasal application.
Lentiviral vectors can also offer both treatment and protection against HIV (Figure 1).8,9
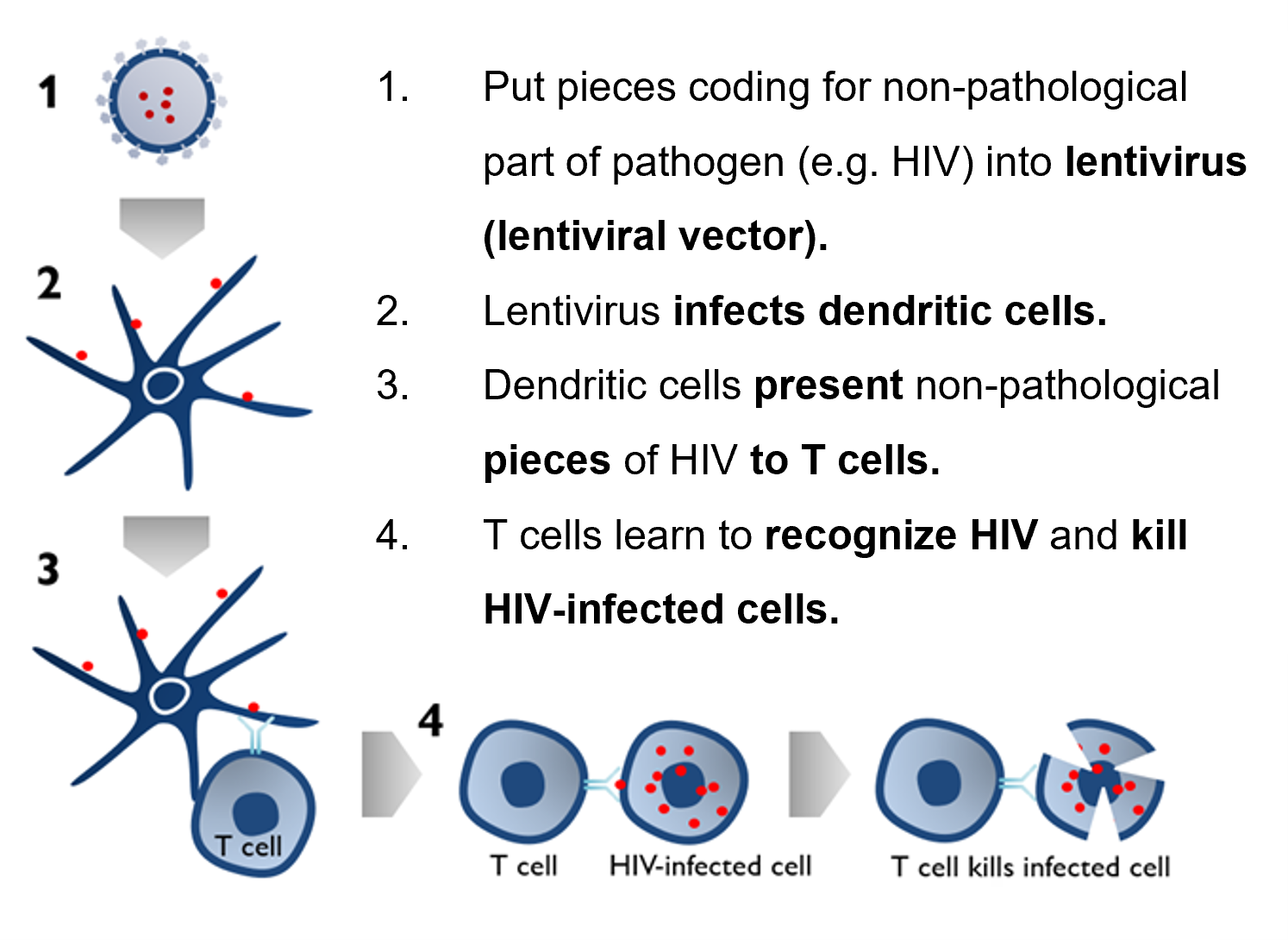
Figure 1. Lentiviral vector HIV protection. Image Credit: Jinwei Bio
Therapeutic applications
In precision medicine, lentiviral vectors have become essential tools, facilitating possible treatments for a variety of conditions.10
These cover targeted HIV treatment via the targeted down-regulation or ablation of CCR5 expression,11 and the creation of tumor-specific T cells for cancer immunotherapy.12,13
Lentiviral vectors, which can infect dendritic cells, offer a method to create T cells that are targeted to several tumor antigens for the elimination of specific tumors.
This successful technology provides multiple benefits compared to CAR-T cell therapy as it can be deployed through a simple injection instead of needing complicated manipulation and harvesting procedures and offers a natural and controlled immune response with no risk of triggering a cytokine storm.
Lentiviral vector-based treatments also have the additional advantage of giving the patient some protection against the growth of tumors in the future, offering cancer prevention along with treatment.
Lentiviral gene therapy based on stem cells also appears to be a solution to life-long treatment for many inherited diseases, such as hemophilia A,14 and Wiskott-Aldrich syndrome.15 This will be established by long-term research on patients who have received gene therapy.
Lentiviral vectors which target dendritic cells are thus an unrivaled technology with the ability to solve many important unmet needs for efficient and safe T-cell vaccines, life-long cures through the accurate alteration of genes and cancer treatments that are better tolerated.
References
- Lundstrom K. Diseases 2018;6(2):42
- Coutant F, et al. PLoS One. 2012;7(11):e48644
- Yang E, et al. Acta Biochim Biophys Sin (Shanghai). 2015 Aug;47(8):588-96.
- Wen Ku M, et al. Molecular Therapy 2020;28,:1772-1782
- de Wispelaere M, et al. PLoS Negl Trop Dis. 2015 Oct 5;9(10):e0004081.
- Ku M-W, et al. Cell Host and Microbes 2021; 29,2:236-249
- Ku M-W, et al. bioRxiv 2021.02.03.429211; doi: https://doi.org/10.1101/2021.02.03.429211
- Beignon AS, et al. J Virol. 2009 Nov;83(21):10963-74./’
- Di Nunzio F, et al. Vaccine. 2012 Mar 28;30(15):2499-509.
- Milone MC, O'Doherty U. Leukemia. 2018 Jul;32(7):1529-1541.
- Haworth KG, et al. CCR5-edited gene therapies for HIV cure: Closing the door to viral entry. Cytotherapy 2017;19(11):1325 1338.
- Haas AR, et al. Mol Ther. 2019 Nov 6;27(11):1919-1929.
- Frank AM, et al. Blood Adv. 2020 Nov 24;4(22):5702-5715.
- Spencer HT, et al. Haemophilia 2016;22(5):66 71
- Aiuti A, et al. Science. 2013 Aug 23;341(6148):1233151.
- Jinwei Bio. http://www.jinweibio.com/
About Jinwei Bio
 Shanghai Jinwei Biotechnology Co., Ltd. (Jinwei) is a biotechnology company dedicated to pursuing innovative technology to develop cures for people with serious and life-threatening diseases.
Shanghai Jinwei Biotechnology Co., Ltd. (Jinwei) is a biotechnology company dedicated to pursuing innovative technology to develop cures for people with serious and life-threatening diseases.
Jinweihas obtained exclusive licenses of multiple patents and other intellectual property owned by the Pasteur Institute and Theravectys for development, manufacture and commercialization of therapeutic products based on lentiviral vectors.
We offer lentiviral vector development, manufacturing and analytical testing for clinical and commercial supply. Our services include Process and Analytical Methods Development, Full GMP Production, with USP and DSP, Fill & Finish, Quality Control & Quality Assurance and Regulatory Support.
The GMP production facility located in Shanghai is dedicated to provide safe and reliable viral vector-based products and CMO/CDMO services, helping to translate early stage research into commercially viable therapies.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.