The development of novel biopharmaceuticals, such as antibodies, is a complex process in the biomanufacturing of therapeutic proteins, and Cell Line Development is a critical component of this process.
Mammalian cells are preferred for protein production as they possess molecular mechanisms that are similar to human cells and can perform necessary protein modifications such as glycosylation.1
In the development of a mammalian cell line, several desirable attributes are required to meet the criteria specified by regulatory bodies such as the ICH, FDA, and EMA.
These agencies require that cell lines for therapeutic use must be cloned from a single-cell progenitor to ensure genetic and phenotypic consistency which require proof of monoclonality.1,2
Five important features desired from a host cell line are:
- Productivity: Must generate high product yields
- Scalability: Grow robustly
- Stability: Be able to grow in serum-free, chemically defined conditions
- Quality: Desired product quality
- Monoclonality: Well-defined clonal cell lines derived from a single cell
Selecting a cell line that possesses these five key characteristics is a challenging task that often results in a bottleneck in the biopharmaceutical production process.
Traditionally, single clones were isolated using the resource-intensive method of limiting dilution. However, advancements in technology, such as fluorescent activated cell sorting (FACS), colony picking, and single-cell printers, have made the selection process more efficient.
After isolation, clones are imaged using cell-in-well imagers and evaluated for productivity before being grown in small-scale bioreactors for batch production.
With the rapidly growing demand for biotherapeutics, there is a pressing need to streamline the development workflow and reduce the time and resources required for cell line development.
Cyto-Mine® leverages the established picodroplet technology to expedite the creation of cell banks that consist of high-producing clones derived from single cells with assured monoclonality.
This article showcases how the Cyto-Mine® IgG secretion assay assesses the productivity of hundreds of thousands of single cells encapsulated in highly uniform picoliter droplets, referred to as "test tubes" while ensuring clonality verification.
Aims and objectives
This article will demonstrate how Cyto-Mine®:
- Identifies high-producing clones from a mixed population
- Accurately identifies clonality
- Significantly shortens the Cell Line Development workflow using a single fully integrated instrument
Box 1.
The Cyto-Mine® Single Cell Analysis and Monoclonality Assurance System overcomes the limitations of current technologies by screening hundreds of thousands of individual cells for the secreted target protein and then isolating and dispensing the highest producers with high viability to microplate wells (Figure 1).
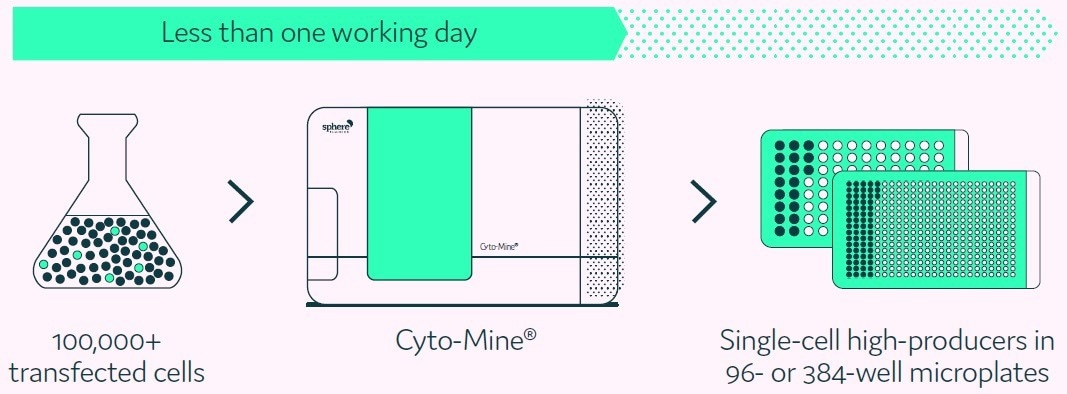
Figure 1. Cyto-Mine® proprietary technology finds and isolates high-producer clones from complex cell populations. Image Credit: Fluidic Sciences and Sphere Bio
Methods
Cyto-Mine® mimics the limiting dilution and productivity screening processes but in a considerably more efficient, higher throughput, and fully automated fashion (Box 1).
Single cell encapsulation
Cells are encapsulated into 300 pL picodroplets of desired culture media using Poisson distribution parameters at a dilution level that maximizes the number of picodroplets carrying just a single cell.
Antibody secretion assay
Next, the cells are incubated to enable the secreted target protein to accumulate inside the picodroplet, which is captured and detected by animal origin free (AOF) IgG Methods detection reagent present in the culture medium (Box 2). The miniaturized scale means that secreted IgG can be quantified after just 0.5 to 2 hours.
Sorting
The highest-secreting single cells are then sorted for collection based on fluorescence intensity.
Clonality assurance
Before dispensing, the encapsulated cells are scanned many times as the picodroplet passes through the microfluidic channels to ensure clonality. Figure 2 depicts the integrated steps of the Cyto-Mine® process
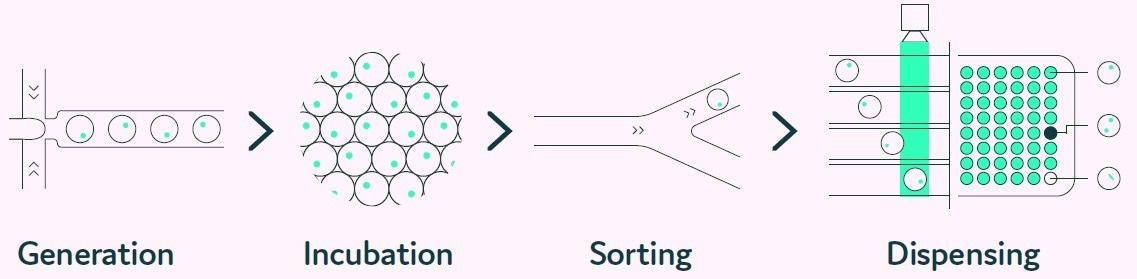
Figure 2. The Cyto-Mine® workflow integrates the screening, sorting, isolation and verification of high-secreting clones intoa fully automated process. Image Credit: Fluidic Sciences and Sphere Bio
Box 2
Antibody secretion assay
The Cyto-Mine® system provides unique capabilities through its ability to measure the specific IgG production rate of individual cells.
The process starts with a cell population that requires no prior modification, simply mixed with the appropriate AOF detection reagents, including donor and acceptor probes labeled with fluorescence.
The cells are loaded onto Cyto-Mine® and subjected to an in-situ incubation, where IgG secreted by the cells accumulates within the picodroplet. The detection probes bind to the secreted IgG, leading to a change in fluorescence due to FRET.
Cyto-Mine® then measures the fluorescent signal produced and converts it into a quantitative output.
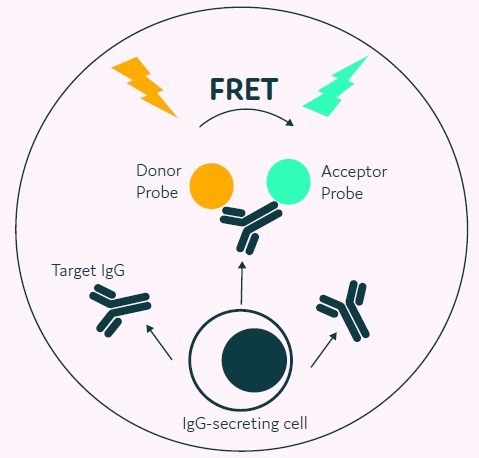
Figure 3. The Cyto-Mine® picodroplet-based IgG secretion assay. A customized pair of IgG-specific AOF fluorescent probes are trapped within each picodroplet. IgG secreted from the encapsulated cell is recognised by the detection probe pair forming a 3-body FRET complex that induces a fluorescent signal. Image Credit: Fluidic Sciences and Sphere Bio
Results
Antibody secretion assay
The Cyto-Mine® IgG secretion assay was validated by creating five different populations of picodroplets, each with a different human IgG concentration ranging from 0 to 20 mg/L. These populations were then combined and analyzed using the Cyto-Mine® system.
The assay was able to accurately distinguish between the different IgG titers, with a working range of up to 20 mg/L, equivalent to a maximum specific productivity of 144 pg/cell/day with a 1-hour incubation.
The results were demonstrated in a standard titration curve obtained from Cyto-Mine® scatter plot data, as shown in Figure 5.
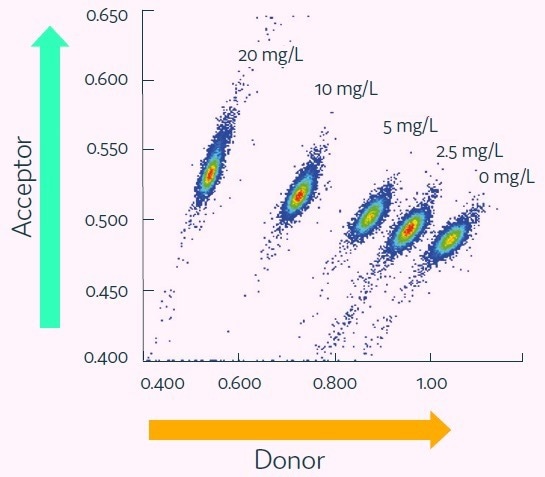
Figure 4. Cyto-Mine® Scatter Plot. Large numbers of individual picodroplets were loaded with the indicated concentrations of human IgG and then resolved using Cyto-Mine® AOF IgG secretion assay and analysis. Image Credit: Fluidic Sciences and Sphere Bio
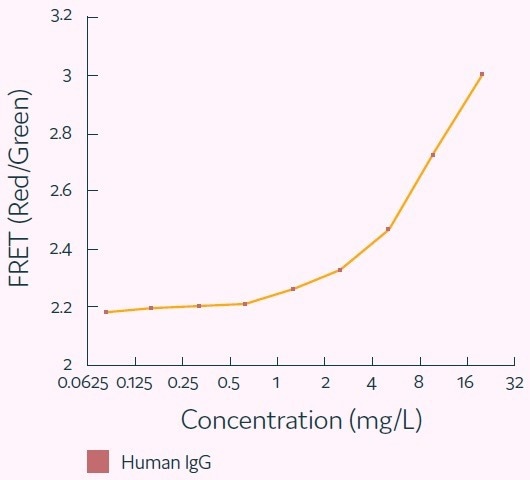
Figure 5. Standard human IgG titration curve derived from Cyto-Mine® AOF IgG secretion assay and analysis. Image Credit: Fluidic Sciences and Sphere Bio
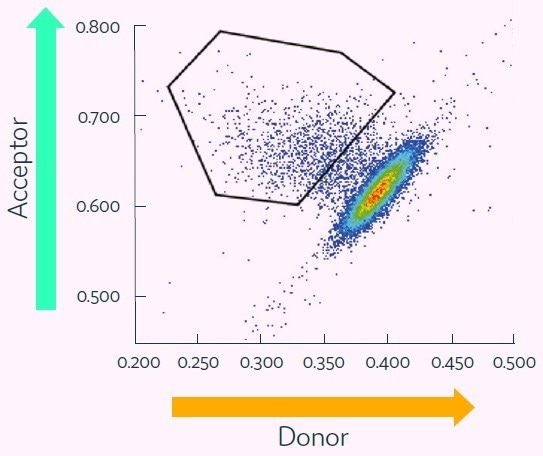
Figure 6. Cyto-Mine® Scatter Plot of FRET signal generated from picodroplet-encapsulated CHO cells incubated with Cyto-Mine® human IgG-specific AOF detection reagent. Image Credit: Fluidic Sciences and Sphere Bio

 Download the full paper
Download the full paper
About Fluidic Sciences and Sphere Bio
Fluidic Sciences develops transformative in‑solution technologies for protein interaction analysis. Its flagship Fluidity One‑M instrument leverages Microfluidic Diffusional Sizing (MDS) to measure binding affinity, stoichiometry, size, and concentration without immobilization - directly in complex backgrounds such as serum, plasma, and lysate.
Sphere Bio is a brand of Fluidic Sciences. Its technology develops and manufactures single‑cell analysis and monoclonality assurance systems that enable researchers to find, analyze, and isolate the most valuable cells with speed and precision. Its proprietary picodroplet microfluidics and Cyto‑Mine® Chroma multiplexing platform power applications across antibody discovery, cell line development, cell engineering, and cell therapy.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.