This article discusses how Cyto-Mine® can be used to screen complex populations of primary B cells and hybridomas by employing a miniaturized, high-throughput, homogeneous secretion assay for the analysis and identification of antigen-specific clones for sorting and collecting to microplates.
Antibody-derived biologics have become a key area of modern medicine, especially regarding the struggle against autoimmune diseases and cancer.
Highly efficacious immunoglobulin-based drugs have been developed naturally via the antibody-producing B cells of the mammalian immune system, although finding rare cells with the right characteristics has always been challenging.
However, recent developments in single cell gene sequencing and recombinant immunoglobulin expression have enabled direct analysis of native B cells, thus allowing much more of the immune system diversity to be interrogated.
This has expanded the potential for deep-mining the repertoire of any mammalian antibody-producing cell type. At present, semi-automated technologies, including colony picking, cell sorting, and cell-in-well imagers, are utilized in combination to screen and isolate rare antigen-specific cells.
The CytoMine® Single Cell Analysis and Monoclonality Assurance System is the first completely integrated platform specifically constructed for biopharma that enables the following:
- The screening of millions of primary B cells or hybridomas for secreted immunoglobulin
- The identification and sorting of the antigen-specific candidates
- The gentle dispensing of these candidates to 96- or 384-well microplates with visual evidence of cell number
A summary of this process is displayed in Figure 1.
The study outlined in this study outlines a method by which the Cyto-Mine® antigen-specific assay analyses heterogeneous B cell and hybridoma populations to identify target-specific variants in a high-throughput manner.
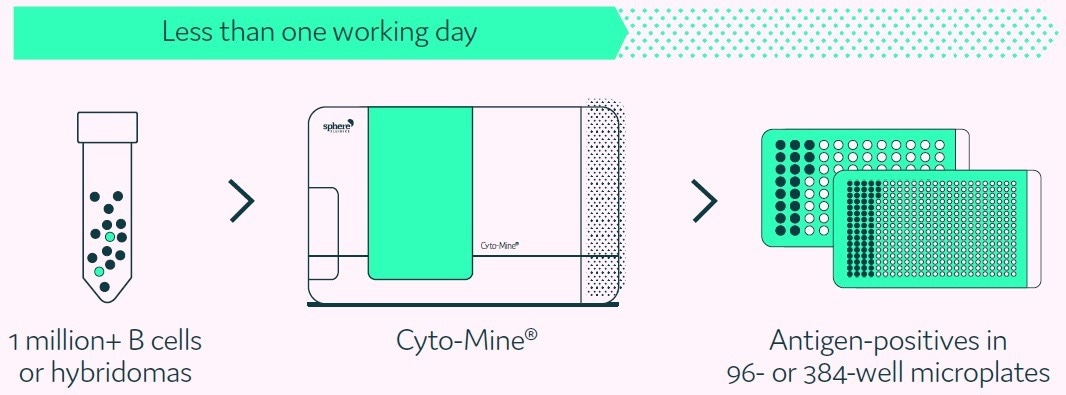
Figure 1. Cyto-Mine® proprietary technology finds and isolates high-producer clones from complex cell populations. Image Credit: Fluidic Sciences and Sphere Bio
Methods
Cyto-Mine® Workflow
The B cell or hybridoma cell population of interest is placed into the appropriate culture medium and is supplemented with the preferred antigen-based detection reagents. Following this, the mixture is pipetted into a Cyto-Cartridge®, which is subsequently loaded into Cyto-Mine®.
The number of cells per picodroplet can be altered as per the user’s requirement, e.g., one cell per picodroplet if single-cell cloning is essential, or more than one cell per picodroplet if the population size exceeds 1 million cells, with the latter leading to the collection of enriched mini-pools of cells rather than single cells.
Cyto-Mine® then incubates the cells in situ to allow immunoglobulin secretion into the picodroplets. The miniaturized format enables an ultrasensitive, rapid assay of typically 0.5 to 2 hours, depending on the production rate of the cell population.
Subsequently, antigen-positive hits are sorted for picodroplet by picodroplet collection. Figure 2 provides a summary of the integrated stages of the Cyto-Mine® antigen-specific assay process.
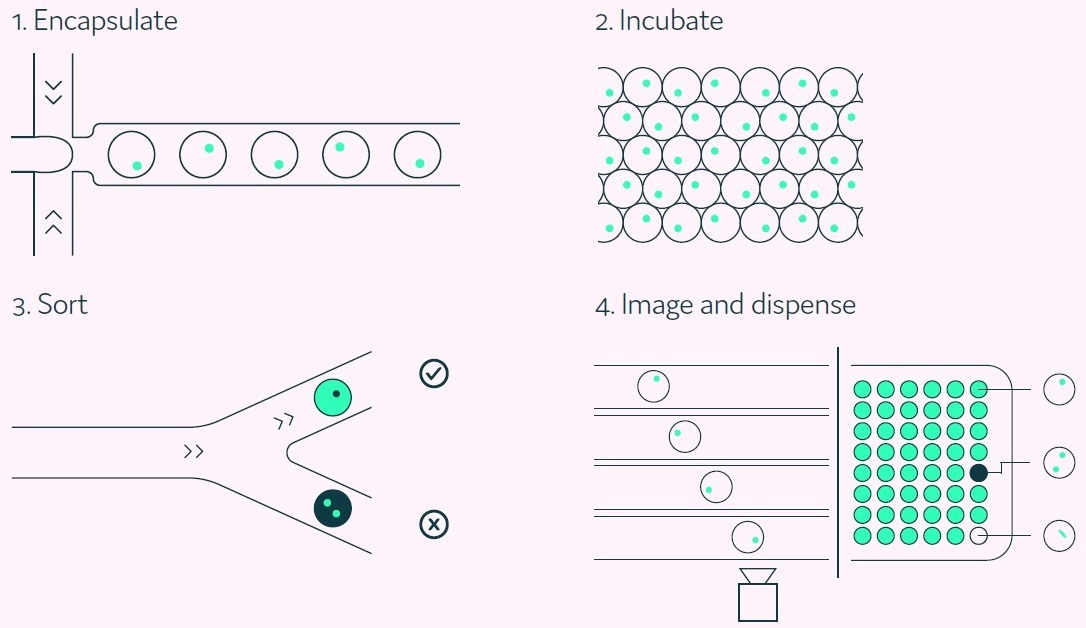
Figure 2. The Cyto-Mine® workflow integrates the screening, sorting, isolation and verification of antigen-specific clones into a fully automated process. Image Credit: Fluidic Sciences and Sphere Bio
Cyto-Mine® antigen-specific assay
A major enabling component of Cyto-Mine® is its capacity to analyze the secreted immunoglobulins of millions of cells for antigen specificity while keeping the cells in a viable state without any prior modification. Figure 3 displays the method by which positives are reported.
The detection probes consist of a fluorescently-conjugated antigen probe and an immunoglobulin-specific acceptor probe. These bind to the secreted antigen-specific immunoglobulin initiating a FRET-mediated shift in fluorescence.
Following this, Cyto-Mine® measures the produced fluorescent signal and converts this to a quantitative output. This acceptor probe allows simultaneous screening for immunoglobulin isotype and antigen specificity.
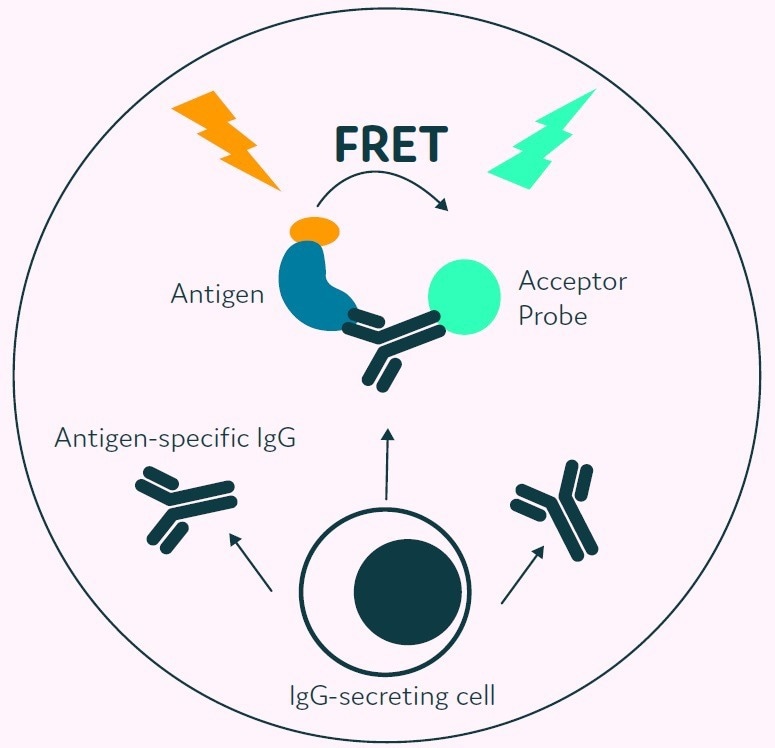
Figure 3. The Cyto-Mine® picodroplet-based antigen-specific assay. This model shows the standard assay to screen for antigen-specific IgG. Antigen-specific IgG secreted from the encapsulated cell during incubation is recognized by both the antigen and the IgGspecific acceptor probe forming a 3-body FRET complex that induces a fluorescent signal. Image Credit: Fluidic Sciences and Sphere Bio
Results
Antigen-specific assay titration curve
To verify that the Cyto-Mine® antigen-specific assay is quantitative, titration experiments were conducted, which utilized human TNF-alpha as a model antigen.
Cell culture medium was spiked with five distinct concentrations of anti-human TNF-alpha IgG up to 20 μg/mL (equivalent to 133 nM) and subsequently encapsulated into picodroplets that contain TNF-alpha and IgG-specific detection probes.
The five populations of picodroplets were pooled and subjected to the Cyto-Mine® process to measure antigen specificity.
The results, displayed in Figure 4, demonstrate how the various titers were resolved as discrete populations. The titration curve presented in Figure 5 shows that the assay is quantitative and specific.
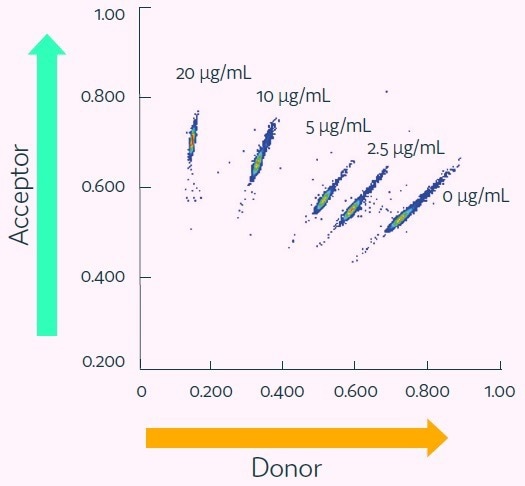
Figure 4. Cyto-Mine® Scatter Plot. Large numbers of individual picodroplets were loaded with the indicated concentrations of anti-human TNF-alpha IgG and then resolved using the Cyto-Mine® antigen-specific assay and analysis. Image Credit: Fluidic Sciences and Sphere Bio
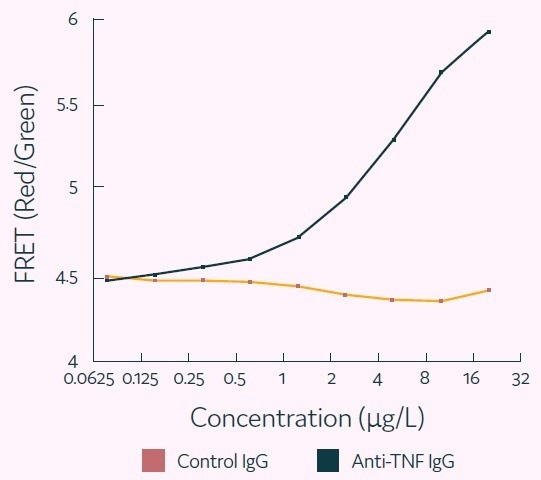
Figure 5. Representative titration curve generated using the Cyto-Mine® antigen-specific assay. In this example, the control (irrelevant) IgG confirms specificity of the assay for human TNF-alpha. Image Credit: Fluidic Sciences and Sphere Bio
Screening hybridomas for antigen-specific clones
As part of the experiment, a hybridoma fusion produced from a mouse immunized with human TNF-alpha was put through Cyto-Mine® to identify TNF-alpha-specific clones.
The cell population was mixed with fluorescently-conjugated human TNF-alpha and acceptor probe against mouse IgG-Fc, and run through the Cyto-Mine® antigen-specific assay workflow.
Figure 6 displays the results, which exhibit a population of cells with a high acceptor-to-donor fluorescence ratio, which have been gated for collection.
The bright oval-shaped cluster in the lower-right corner represents the considerable majority of negative data points. These consist of empty picodroplets and picodroplets that contain either non-producers or non-antigen-specific cells.
This study involved the gating polygon being set to sort all antigen-positive clones, but this is completely customizable to suit experimental requirements.
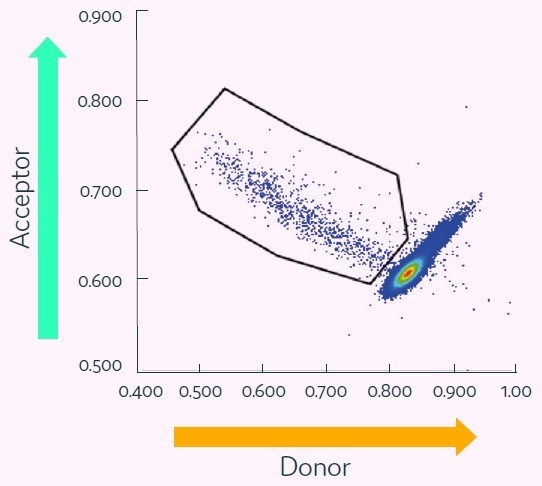
Figure 6. Cyto-Mine® Scatter Plot of FRET signal generated from hybridomas encapsulated in picodroplets and screened for secretion of human TNF-alphaspecific IgG. Image Credit: Fluidic Sciences and Sphere Bio
Conclusions
These results demonstrate the power of Cyto-Mine® for screening large cell populations to identify, sort, and isolate high-value clones. The assay is quantitative within the range of immunoglobulin typically generated by standard biopharmaceutical production cell types.
Cyto-Mine® and its picodroplet-based IgG secretion assay provide the following key advantages:
- Simplicity: A seamless automated process with only one step, which eliminates the requirement for numerous instruments
- Quality: The unique ability to mine and isolate rare, single, viable, high-producing cells
- Sterility: It is sterile end-to-end, with a disposable and AOF process
- Speed and efficiency: It enables multiple projects to be run concurrently and for users to free up time for other tasks
- Miniaturization: It allows pL-level high-throughput screening with little use of assay reagents
- Traceability: It provides storable images to prove single cell status at the time of dispensing, delivering the required documented evidence of monoclonality

 Download the full white paper
Download the full white paper
Acknowledgments
Produced from materials originally authored by Fluidic Sciences and Sphere Bio. The work detailed in this article was partially funded by the UK Government Advanced Manufacturing Supply Chain Initiative (AMSCI) as part of the BioStreamline Project which exists to develop new approaches for the more efficient development and manufacture of next-generation biologics.
About Fluidic Sciences and Sphere Bio
Fluidic Sciences develops transformative in‑solution technologies for protein interaction analysis. Its flagship Fluidity One‑M instrument leverages Microfluidic Diffusional Sizing (MDS) to measure binding affinity, stoichiometry, size, and concentration without immobilization - directly in complex backgrounds such as serum, plasma, and lysate.
Sphere Bio is a brand of Fluidic Sciences. Its technology develops and manufactures single‑cell analysis and monoclonality assurance systems that enable researchers to find, analyze, and isolate the most valuable cells with speed and precision. Its proprietary picodroplet microfluidics and Cyto‑Mine® Chroma multiplexing platform power applications across antibody discovery, cell line development, cell engineering, and cell therapy.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.