As researchers race to identify new therapeutics, streamlined antibody discovery processes have never been more crucial, and the need for speed and efficiency is paramount.
The development of therapeutic antibodies is dependent on a comprehensive examination of B cell repertoires or hybridoma populations. Yet, traditional screening procedures are time-consuming and expensive.
This presents significant obstacles in the discovery of lead candidates for antibody modification and therapeutic candidate selection.
This article describes a robust and quick single B cell screening strategy for identifying antibodies with the necessary antigenic selectivity without the arduous requirement for conventional hybridoma screening.
Cyto-Mine® Single Cell Analysis System from Fluidic Sciences and Sphere Bio utilizes patented pico-droplet-based technology to harvest and analyze antibodies directly from whole B cell repertoires, therefore conserving cell viability and significantly enhancing efficiency.
Researchers can detect antibody-secreting cells and extract rare cells secreting antigen-specific antibodies within just two days using this technology, greatly reducing the time required for treatments to reach the market.
Challenges in the antibody discovery process
Generally, antibody discovery begins with the creation of hybridomas by fusing a B cell population with a myeloma cell population or using technologies such as phage display. All these procedures need several iterative examinations of antibody libraries to uncover leads.
The leads are subsequently characterized, modified, and advanced to pre-clinical research. Despite technical and methodological advancements, there are still significant challenges to overcome.
First, hybridoma generation is time-consuming, expensive, and inefficient, as this approach may result in missing the rare antigen-binding antibody with the desired characteristics.
During hybridoma formation, many B cells die in development and cannot achieve effective fusion, resulting in the potential loss of vital antibody-secreting cells.
Second, conventional methods only examine a fraction of the cell repertoire. In the hybridoma scenario, a significant portion of the B cell repertoire is discarded during hybridoma formation; consequently, only a portion of the population is screened, and the most effective candidate molecules cannot be identified.
Considering the population of target cells can be as low as 0.001% of the original ~40 million cells harvested, this is a significant challenge to address.
The development of technology that can provide screening of the whole B cell population is paramount to ensure that key target-specific antibody-secreting cells are not missed.
High-throughput screening (HTS) remains the most successful lead generation strategy; when applied to the development of therapeutic antibodies, it reduces the time required to identify and isolate the rare antigen-specific, antibody-secreting cells from a population of millions.
Circumventing hybridoma fusion and phage display is often accomplished by screening the isolated B cells directly using flow cytometry.
Flow cytometry has the advantage of being very high-throughput, and antibodies secreted by B cells can potentially be screened using cold capture, a technique used to prevent the full secretion of antibodies by trapping them at the cell surface.
However, this is a representation rather than a direct measurement of the antibody secretion profile by a single cell.
Flow cytometry can also be harsh on cells, particularly primary cells, and may change cell viability and function.
Alternative screening procedures include ELISA and Elispot; however, these techniques are often conducted manually, making it prohibitively expensive and time-consuming to test huge populations.
After several rounds of screening and selection, the positive cells must be sub-cloned into monoclonal populations (lead panels) using semi-automated methods such as cell-in-well imagers and cell sorting; this multi-step approach provides complexity and hands on-time, delaying the process even more.1
Achieving improved process efficiency with microfluidics
Microfluidics, specifically droplet microfluidics, has contributed to the advancement of HTS. Microfluidic systems conduct complex multi-step assays with high reliability, cost-efficiency, and throughput in a picoliter volume water-in-oil emulsion droplet (picodroplet) format.
Picodroplets provide the benefits of miniaturization and automation, hence enhancing laboratory efficiency by permitting quick, high-throughput research to examine larger repertoires and identify more functional features.2
Cyto-Mine® provides a robust, high throughput platform for screening whole B cell repertoires for antigen-specific antibody-secreting B cells via pico-droplet-based technology. The automated platform can screen, select and distribute antibody-secreting cells with antigen specificity in only two days.
Using a bulk analysis to enrich B cells that exhibit the desired antibody, followed by an antigen detection assay to identify antigen-specific antibodies, constitutes the two-step screening procedure.
The extracted antigen-specific antibody-secreting cells are subsequently distributed as a pooled population or as individual cells onto microtiter plates with 96 or 384 wells for further investigation.
Encapsulation in pico-droplets assures that the concentration of chemicals released by a cell rapidly rises and shields single cells from shear stress, therefore overcoming some of the most significant obstacles of flow cytometry and fluorescence-activated cell sorting.
In this two-step approach, the versatility of Cyto-Mine® permits the encapsulation of cell populations inside pico-droplets for the first screening round (shown in Figure 1B) and the encapsulation of single cells for the second screening round (illustrated in Figure 1A).3
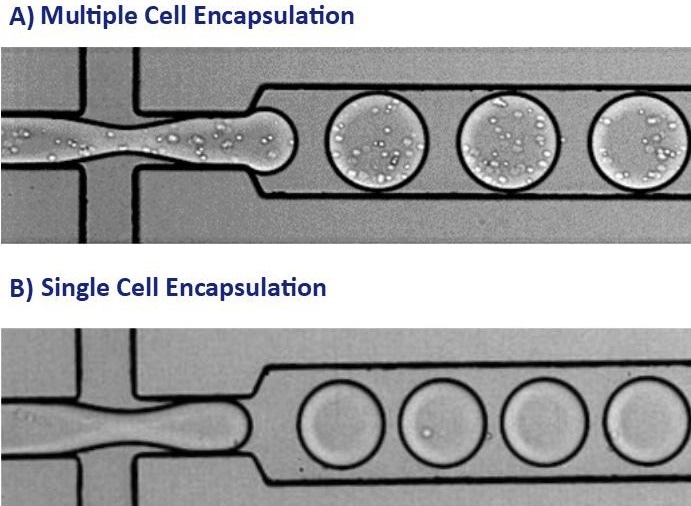
Figure 1. These images show the encapsulation of single cells or multiple cells per picodroplet. A) A large population of cells (greater than 1 million) were diluted to a concentration of 1x108 cells/mL in medium resulting in multiple cells per picodroplet. B) Cells were diluted to a concentration of 1x106 cells/mL to obtain a population of mostly 1 cell per occupied picodroplet. Image Credit: Fluidic Sciences and Sphere Bio
Overall, Cyto-Mine® addresses some of the major challenges faced in the antibody discovery workflow, which are:
- Flexibility - Adaptable assay design for specific needs
- Measurement - Semi-quantitative assays of antibody secretion
- Sensitivity and specificity - Detect antibodies of interest
- Efficiency - Screen the entire cell population
- Viability - Maintain high levels of cell viability
- Speed - Reduce total workflow timelines
References
- Zhang, H., Wilson, I. A., and Lerner, R. A. (2012). Selection of antibodies that regulate phenotype from intracellular combinatorial antibody libraries. Proceedings of the National Academy of Sciences, 109(39), 15728-15733. doi:10.1073/pnas.1214275109.
- Matuła, K., Rivello, F., and Huck, W. T. (2020). Droplet Microfluidics: Single‐Cell Analysis Using Droplet Microfluidics (Adv. Biosys. 1/2020). Advanced Biosystems, 4(1), 2070012. doi:10.1002/adbi.202070012.
- Josephides, D. et al. (2020). Cyto-Mine: An Integrated, Picodroplet System for High-Throughput Single-Cell Analysis, Sorting, Dispensing, and Monoclonality Assurance. SLAS TECHNOLOGY: Translating Life Sciences Innovation. https://doi. org/10.1177/2472630319892571

 Download the full paper
Download the full paper
About Fluidic Sciences and Sphere Bio
Fluidic Sciences develops transformative in‑solution technologies for protein interaction analysis. Its flagship Fluidity One‑M instrument leverages Microfluidic Diffusional Sizing (MDS) to measure binding affinity, stoichiometry, size, and concentration without immobilization - directly in complex backgrounds such as serum, plasma, and lysate.
Sphere Bio is a brand of Fluidic Sciences. Its technology develops and manufactures single‑cell analysis and monoclonality assurance systems that enable researchers to find, analyze, and isolate the most valuable cells with speed and precision. Its proprietary picodroplet microfluidics and Cyto‑Mine® Chroma multiplexing platform power applications across antibody discovery, cell line development, cell engineering, and cell therapy.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.