The use of cannabis and cannabis derivatives in nutritional supplements and medicine has recently grown globally, mostly due to regulatory approval for medicinal use. As more new compounds are released onto the market, accessible techniques are needed to quickly and efficiently analyze product quality.
In the UK, Cannabidiol (CBD) has become popular as a health supplement, and many studies have reported the benefits of analgesic anti-depressants.
In its natural form, CBD is derived from the Cannabaceae genus of flowering hemp, and the essential oils that form the body of the sample matrix are released during the extraction process. This complex matrix provides a challenge when looking to perform product quality testing.
This article outlines how a quality assurance scientist may extract CBD from the starting matrix using a hyphenated technique of CAMAG’s planar chromatography technology and Microsaic’s “point-of-need” mass spectrometer and fluidics handling platform.
Experiment
Three forms of CBD samples were obtained, namely 10 mg capsules, 2.75% hemp oil, and 5% hemp oil droplets. Methanol was added to all samples, and these samples were then injected into HPLC vials and subsequently placed into the CAMAG Automatic TLC Sampler 4.
Narrow bands of samples were applied onto HPTLC plates (HPTLC Si RP-18 F254, 20x10), and Vision CATS software was utilized. Plates were developed via the CAMAG Automatic Developing Chamber 2.
A developing solvent mixture consisting of methanol, water, and acetic acid in a ratio of 70:15:15 was used and subsequently presented using the CAMAG TLC Visualizer 2. Bands were extracted from the developed plate with the use of the CAMAG TLC 2 Interface.
An extraction solvent of methanol and water in a ratio of 80:20 at 0.5 ml/minute was supplied by the Microsaic MiDasTM, and the extracted sample was passed into the Microsaic 4500 MiD®.

Figure 1. System configuration showing the TLC2 interface connected to the 4500 MiD®. Image Credit: Microsaic Systems plc
Results
The developed plates are displayed in Figure 2, together with a CBD standard for comparison. There is good agreement with the developed band around 0.3 rf, providing confidence that the separation was successful.
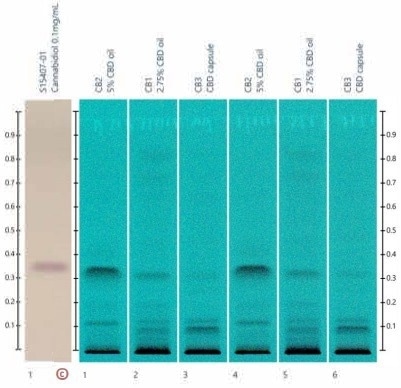
Figure 2. Developed plate showing separated samples. Band at 0.3 rf corresponds well with reported rf value for CBD. Image Credit: Microsaic Systems plc
Mass detection is preferred because it enables direct confirmation to be obtained as well as eliminating expensive and difficult-to-acquire reference standards. Information-rich data containing matrix impurities and co-eluting compounds can also be obtained.
![MaSScope data showing combination of TIC over 50-1400u mass rage and SIM scan @ 315.2. Bottom shows the mass spectrum that is extracted from the TIC *CBD peak (m/z 315.2; [M+H]+).](https://d2jx2rerrg6sh3.cloudfront.net/images/appnotes/ImageForAppNote_4590_45093375498402786109.jpg)
Figure 3. MaSScope data showing combination of TIC over 50-1400u mass rage and SIM scan @ 315.2. Bottom shows the mass spectrum that is extracted from the TIC *CBD peak (m/z 315.2; [M+H]+). Image Credit: Microsaic Systems plc
![Mass spectrum of extracted band @ 0.3 rf CBD 315.2 m/z; [CBD+H]+.](https://d2jx2rerrg6sh3.cloudfront.net/images/appnotes/ImageForAppNote_4590_45093375503518527073.jpg)
Figure 4. Mass spectrum of extracted band @ 0.3 rf CBD 315.2 m/z; [CBD+H]+. Image Credit: Microsaic Systems plc
After the developed plate was transported to the TLC Interface 2, the targeting laser was aligned with the band located at 0.3 rf. The sample was subsequently extracted and passed through the solvent stream and through an inline filter to reach the MiD mass detector.
The mass spectrometry data from the developed plate is displayed in Figure 3. A good response is evident from a full scan total ion count (TIC) over the whole mass range (50-1400 m/z) in tandem with a SIM acquisition that is run simultaneously (MaSScope; 315.2 m/z ).
The signal that corresponds to [CBD+H]+ in the mass spectrum can be observed in Figure 4, which proves the MiD’s sensitivity and flexibility to run two methods simultaneously.
Conclusions
CAMAG planar chromatography equipment was employed in combination with Microsaic point-of-need technology to extract mass data from popular high-street health supplements containing CBD.
The MiD’s accessibility means it is highly compatible with CAMAG planar technology. The resultant usability means that the time taken from setup to analysis is reduced, allowing mass analysis of developed plates to occur in line with established workflows.
As Cannabis is increasingly accepted for use in both medicine and nutritional supplements, versatile methods are necessary to tackle these challenges. Microsaic Systems Plc and Omicron Research Ltd are committed to bringing these solutions to the point of need.
Acknowledgments
Produced from materials originally authored by Microsaic Systems Plc. The original authors wish to thank Omicron Research Ltd and the University of Westminster for access to chromatography equipment and application support.
About Microsaic Systems
Founded in 2001, Microsaic Systems plc (AIM: MSYS) develops real-time, point-of-need mass spectrometers. Microsaic offers fast, accurate cutting-edge solutions to multiple industries across the world.
Core products, such as the compact MiD series of mass detectors, are designed to integrate seamlessly with a wide range of third-party OEM equipment or used as a standalone system. At the forefront of our design ethos is to deliver fast, easy to use, powerful mass spectrometry (MS) performance.
Patented chip-based MS technology and intuitive software enables real-time data generation at the point-of-need, not just in a centralised laboratory. Designed for both pharmaceutical and biopharmaceutical applications, continuous data is accessible at any stage of your workflow.
Over 20 years’ experience in mass spectrometry, microfluidics, vacuum systems, analytical processes, and miniaturised instrumentation has led to the development of our outsourced services. Laboratory, engineering, and monitoring issues can now be solved with a world-class team of chemists, physicists, and engineers by your side.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.