Since its introduction in the 1980s, endocrine therapy with tamoxifen has been the first-line treatment for breast cancer in estrogen-receptor-positive men and women. In addition to being valuable as adjuvant treatment following surgery, radiation, and/or chemotherapy, tamoxifen is also used to treat metastatic breast cancer and reduce the overall incidence in women identified as high-risk.
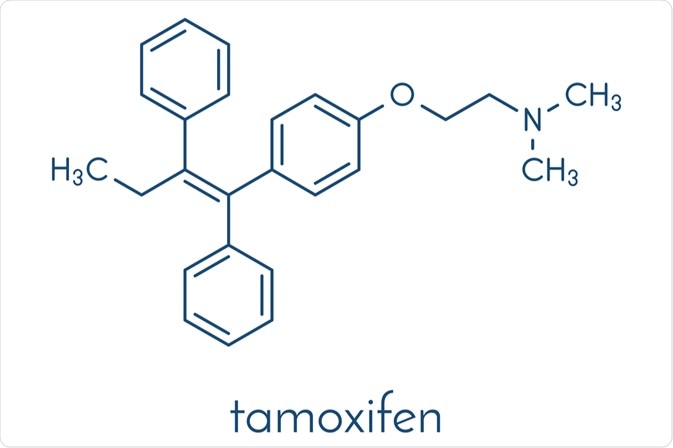
Image Credit: Studiomolekuul / Shutterstock.com
Mechanism of action
Categorized as a classic “pro-drug,” tamoxifen requires metabolic activation in order to elicit its pharmacological effects. Importantly, numerous studies have revealed CYP2D6 as the rate-limiting enzyme responsible for converting the pharmacologically inactive tamoxifen to its metabolites endoxifen (4-OH-N-desmethyl-tamoxifen) and 4-hydroxytamoxifen.
After the metabolites are generated, they interact with the estrogen receptor in target tissues including both breast and non-breast tissues to produce a complex phenotype, resulting in both agonist and antagonist effects.
CYP2D6
The CYP2D6 enzyme is critically involved in the metabolism of approximately 20-25% of all developed drugs and more than 48 different clinical substrates (drugs) for this enzyme have been identified, which include β-blockers, antidepressants, opioid analgesics, antiarrhythmics, and antipsychotics.
The CYP2D6 gene is highly polymorphic; in fact, there are currently 63 different major alleles known, each of which is associated with a final gene product with increased, decreased, or entirely non-existent function. Once translated, the different CYP2D6 phenotypes associated with these alleles include poor (PM), intermediate (IM), extensive (EM), and ultrarapid (UM) metabolizers.
The phenotype distribution that results has been shown to vary between ethnic groups. For example, people exhibiting 2 of the approximately 20 known alleles show poor metabolizing action, with 7–10% of these poor metabolizers present in the European and North American Caucasian population.
In contrast, individuals with the UM phenotype carry both gene and functional allele duplication, which results in a higher CYP2D6 expression and enzyme activity. This phenotype is relatively infrequent in Caucasians and Asians, but up to 30% is seen in Ethiopians.
Factors affecting treatment outcomes
Despite the existing, wide inter-individual variability in the concentrations of tamoxifen and its metabolites, there was no evidence linking variability in tamoxifen or its metabolite concentrations with patient response or side effects.
There are suggestions that both genetic and environmental factors alter CYP2D6 enzyme activity and in this way affect tamoxifen treatment outcomes. Knowing this might provide a route to individualize the hormonal therapy of breast cancer for patients.
The concept of genetic variations in the gene impacting tamoxifen treatment outcome was formed following tests conducted in breast cancer patients who were taking selective serotonin reuptake inhibitors (SSRIs) to relieve hot flashes. Specifically, because SSRIs inhibit CYP2D6, significantly lower plasma endoxifen concentrations were detected in the studies’ patients.
Some newer SSRIs, such as paroxetine or fluoxetine, have even been shown to have such an effect on the CYP2D6 gene that the EM phenotype was converted to the PM phenotype.
In addition to this, further studies have shown the risk of breast cancer recurrence is higher in patients taking both tamoxifen and an SSRI (e.g. Paxil, Prozac, or Zoloft— the most ‘potent’ CYP2D6 inhibitors) in comparison to those taking only tamoxifen. This recurrence was not increased in those taking the ‘weaker’ antidepressants (e.g. Celexa, Lexapro, or Luvox), did not present with an increased risk of breast cancer recurrence.
It is important to note that despite the evidence for the involvement of the CYP2D6 gene in the metabolism and resultant outcome of tamoxifen therapy, there is still much to learn about the cytochrome P450 pathway considered responsible for the metabolism of tamoxifen.
References:
- http://www.ebmconsult.com/articles/cyp2d6-genetic-polymorphisms-medication-substrates
- http://emedicine.medscape.com/article/1879354-overview
- http://www.nature.com/tpj/journal/v5/n1/full/6500285a.html
- http://www.ncbi.nlm.nih.gov/books/NBK247013/
- https://www.health.harvard.edu/
- http://www.macmillan.org.uk/Cancerinformation/Cancertreatment/Treatmenttypes/Hormonaltherapies/Individualhormonaltherapies/Tamoxifen.aspx
- http://emedicine.medscape.com/article/1762071-overview#showall
Further Reading
Last Updated: Feb 25, 2023