Calcium is the most abundant mineral in the human body, making up about 2% of the total body weight. Some of the roles calcium plays in the body are described below.
Biological role of calcium
The main role of calcium in the body is to provide structure and strength to the skeleton. In the earliest forms of exoskeleton and in shells, the structural rigidity is generally provided by calcium carbonate. In vertebrates such as reptiles, fish, mammals and humans, the structure of the skeleton is mainly provided by a form of calcium phosphate called hydroxyapatite crystals, which are found in collagen. Calcium ions on bone surfaces interact with those present in the bodily fluids, therefore enabling ion exchange, which is essential in maintaining the balance of calcium in the blood and bone.
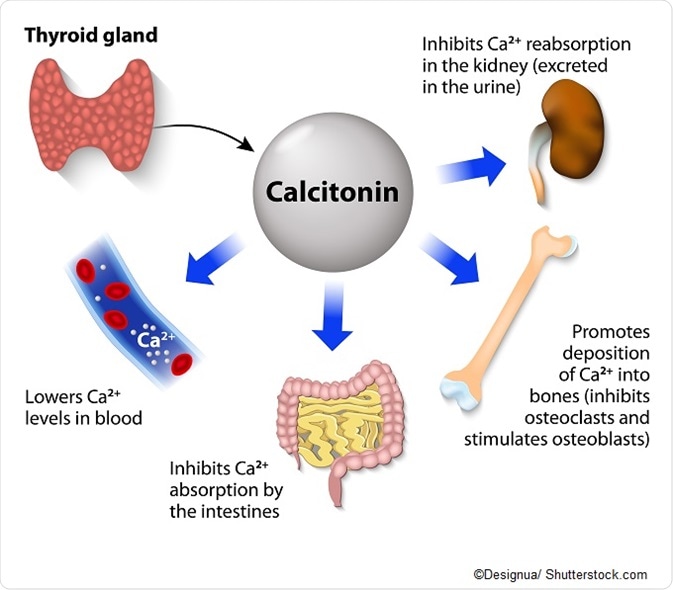
In the extracellular fluid, calcium serves as a reservoir for circulating calcium. This calcium enters the extracellular fluid via the gastrointestinal tract where it is absorbed from the food a person has eaten. Calcium also becomes available through a process called bone resorption, which is the breakdown of bone tissue by cells called osteoclasts.
Calcium circulating in the blood is involved in several vital processes including coagulation, nerve signal transmission, hormone signalling and muscle contraction.
Calcium uses in general
- Calcium may be used as a reducing agent in the process of metal extraction
- Calcium is also used in the production of some metals, as an allying agent.
- Calcium carbonate is used to make cement and mortar and also in the glass industry.
- alcium carbonate is also added to toothpaste and mineral supplements.
- Calcium carbide is used to make plastics and to make acetylene gas.
- Calcium arsenate acts as an insecticide and calcium phosphide can be used as a rodenticide, as well as in fireworks and flares.
- Calcium phosphate is used in animal feed and fertilizers.
- Calcium hydroxide solution is used as blackboard chalk and as Plaster of Paris in its hemihydrate form.
- Calcium gluconate is used as a food additive.
- Calcium stearate is used to make wax crayons, cosmetics, plastics and paints.
Further Reading
Last Updated: Jun 15, 2023