The term angiogenesis denotes the growth of blood vessels from the existing vasculature. This process begins in utero and occurs throughout life, so that no metabolically active tissue in human body is further than a few hundred micrometers from blood capillaries, which is necessary for the exchange of nutrients and metabolites.
In the 1970s, a surgeon Judah Folkman hypothesized that tumor growth depends on angiogenesis, thus the inhibition of this process could be a potential strategy for treating cancers. This groundbreaking prediction was a cornerstone of a new field of research, which resulted in antiangiogenic drugs that are used in modern clinical practice to treat malignant and vascular retinal diseases.
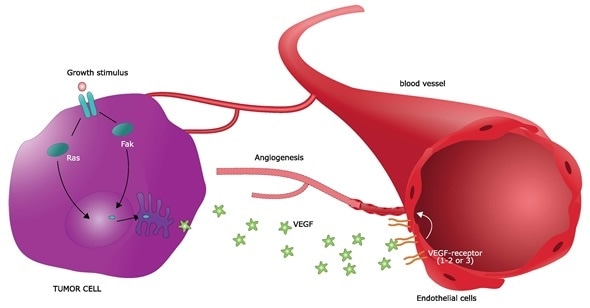
Image Copyright: ellepigrafica, Image ID: 270363338 via Shutterstock.com
Research advancement and proof of concept
The initial hypothesis from Folkman was not immediately accepted by the scientific community at the time, as the dominant paradigm was that the formation of blood vessels is an incidental event unrelated to tumor growth. Still, in 1979 Folkman and his colleagues succeeded in creating a long-term culture of capillary endothelial cells in a medium prepared with cells from a solid tumor.
These endothelial cells were subsequently used to create prototypic reproducible in vitro assays, which remain among the most frequently employed in vitro models for identifying novel stimulators and inhibitors of angiogenesis. Folkman was also a pioneer in developing in vivo models of angiogenesis later in his career.
The discovery of tumor-derived proangiogenic factors followed in the ensuing years. It was shown that most types of parenchymal cells (such as muscle or liver cells) respond to a hypoxic environment by secreting two key angiogenic growth factors – vascular endothelial growth factor (VEGF-A) and fibroblast growth factor 2 (FGF-2). These insights paved the way for the identification of a dozen auxiliary angiogenic factors from both tumor and non-tumor tissues.
As blood vessels in most tissues show low turnover rates, researchers hypothesized that certain endogenous inhibitors of angiogenesis act to counterbalance signals that can trigger persistent vascular growth. Langer and his colleagues were first to purify one of these factors from cartilage that successfully blocked tumor-induced blood vessel growth in the corneal tissue.
Additional inhibitors of angiogenesis were discovered shortly after; for example, steroids (such as cortisone and dexamethasone) were shown to successfully inhibit endothelial activity both in vitro and in vivo, whereas heparin was found to modulate their angiostatic activity. This was a spark of hope for finding a plethora of therapeutic opportunities in the battle against cancer.
Potential therapies and challenges
Efforts aimed at blocking angiogenesis process in both malignant tumors and eye diseases resulted in the approval of drugs that target vascular endothelial growth factor. Nevertheless, only a small percentage of patients with cancer can experience certain benefits, as some tumors are recalcitrant towards VEGF inhibitors or can develop resistance mechanisms.
Several antiangiogenic drugs have been approved for use in humans, most notably bortezomib and thalidomide for the treatment of multiple myeloma in 2003, and bevacizumab for the treatment of colorectal cancer in 2004. Moreover, recent phase 3 clinical trials in patients with ovarian cancer have shown some beneficial effects of a latter drug.
Still, bevacizumab does not yield satisfactory antitumor effects and survival benefits when applied as a monotherapy in patients with metastatic cancer. Therefore a handful of preclinical studies in different animal tumor models (even those with sarcoma) have unveiled the mechanisms behind the synergistic and additive effects of combining different drugs.
In conclusion, research in angiogenesis still gives us new information and molecular insights regarding vessel branching. Additional bedside-to-bench research studies are still necessary in order to obtain the feedback we need for further refinement and optimization of antiangiogenesis therapy.
Further Reading
Last Updated: Jan 8, 2021