Skip to:
Nanoparticles are small sized particles capable of penetrating vasculature and circulating the body. Since their development, they have been applied to imaging as a way of tagging specific areas of the body or tissue, including in magnetic resonance imaging (MRI), optical imaging, and even ultrasound imaging.
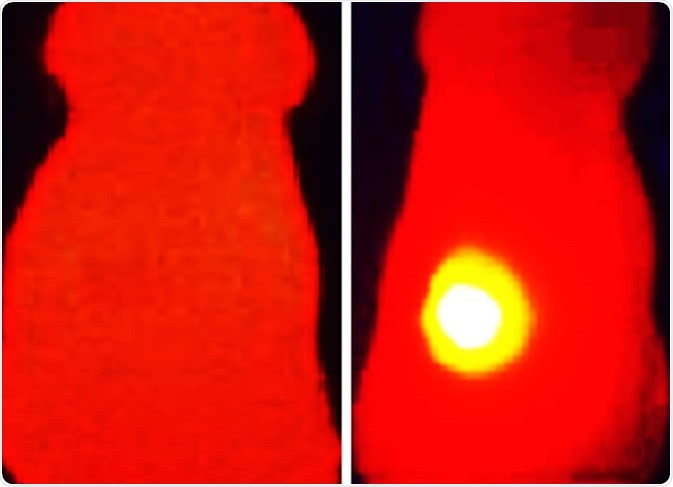
Infrared thermal image (right) shows elevated tumor (yellow) temperature in mice after laser irradiation in with OMV-melanin treated mice. The image on the left shows a mouse treated with OMVs without melanin. Image Credit: Vipul Gujrati / Technical University of Munich
What are Nanoparticles?
One of the most attractive qualities of nanoparticles when considering their application to life sciences are their long retention time and tissue penetration rate. Both these benefits are related to nanoparticle size: whereas larger particulates are recognized and cleared by the reticuloendothelial system following injection, nanoparticles are small enough to escape the reticuloendothelial system and thus survive. Furthermore, their small size means they can penetrate many pore sizes, particularly within tumors.
These properties mean that nanoparticles have, among other uses, been applied to imaging to target particular tissues. Nanoparticles can be engineered to target specific sites with very high specificity. For example, nanoparticles that target cancers have been developed to image the specific location and mass of tumors.
How Do Nanoparticles Improve Image Resolution in Terms of Fluorescence?
As mentioned, nanoparticles can be engineered to target specific sites in the body. Thanks to their capacity to survive and enter the body’s circulatory system, nanoparticles can be injected intravenously and subsequently reach their target destination.
Once the nanoparticle reaches its destination, it may release a functional payload that it carries. For example, some developments have been made in tagging nanoparticles with drugs to treat illnesses in particular parts of the body. In imaging, nanoparticles can be tagged with fluorophores, such as perfluorocarbon emulsion nanoparticles. When the nanoparticle then binds to the receptor or tissue of interest, fluorescence is emitted and contrast is improved. For example, perfluorocarbon emulsion nanoparticles contain a liquid core that enhances contrast in ultrasound images of cardiovascular tissue.
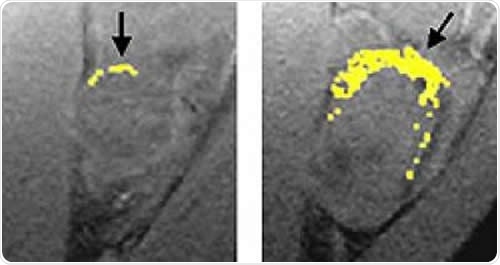
A tumor treated with fumagillin nanoparticles (left) is smaller than an untreated tumor. Nanoparticles containing an image-enhancing metal (yellow) show that the treated tumor has much less blood vessel growth than the untreated tumor. Image Credit: Washington University School of Medicine
Nanoparticles have shown promise in overcoming issues associated with other imaging techniques, even when those techniques are combined. For example, autofluorescence of surrounding tissue has been a big issue for image resolution and in vivo imaging interpretation. Photoluminescent porous silicon nanoparticles have an extended emission lifetime (5-13 µs) compared to tissue autofluorescence signals (<10 ns). Therefore, application of these nanoparticles has been shown to improve signal to background contrast by 50 and 20 fold, in vitro and in vivo, respectively. This form of time-gates imaging can help improve medical imaging while still using conventional imaging techniques.
Magnetic Particle Imaging Methods
Nanoparticles have also assisted in developing novel imaging techniques. Magnetic particle imaging (MPI) is a relatively new technique that detects iron oxide nanoparticle tracers. It is capable of nanomolar sensitivity, image resolution that is independent of depth, and utilizes a safe and stable tracer that can be tracked for weeks. MPI differs from many other medical imaging techniques because it only images the tracer and does not see the tissue itself, unlike MRI and ultrasound.
Despite these advantages, MPI does not have a resolution matching MRI or CT techniques. The spatial resolution of MPI depends on the saturation field of the nanoparticle, its selection strength, and its magnetic relaxation properties. Studies on the nanoparticles rotation time constant reveal that image resolution can be improved by lowering the drive field amplitudes, thereby offering a new perspective on how to improve image resolution and make MPI more competitive compared to traditional medical imaging techniques.
In What Other Settings Can Nanoparticles Improve Image Resolution?
Nanoparticles are being applied to several types of imaging and microscopy techniques, including scanning electron microscopy. For example, digital image correlation (DIC) has been combined with scanning electron microscopy to map surfaces at the nano-scale. However, for DIC to work it needs the material surfaces to have random, isotropic, and high contrast pattern of speckles. This can be provided by gold nanoparticles, which when applied to a sample result in a previously unattainable image resolution of 4 nm/pixel. Here, nanoparticles can be used effectively without large costs, long preparation time, or uneven coverage. In such cases, nanoparticle imaging is not only limited to biological applications but can be applied to material surfaces such as metallic and non-metallic substrates.
Sources
- Liu J., et al. (2006). Nanoparticles as image enhancing agents for ultrasonography. Physics in Medicine and Biology. https://doi.org/10.1088/0031-9155/51/9/004
- Croft L.R., et al. (2015). Low drive field amplitude for improved image resolution in magnetic particle imaging. Medical Physics. https://doi.org/10.1118/1.4938097
- Kammers A.D. and Daly S. (2013). Self-assembled nanoparticle surface patterning for improved digital image correlation in a scanning electron microscope. Experimental Mechanics. https://doi.org/10.1007/s11340-013-9734-5
- Gu, L. et al. (2013). In vivo time-gated fluorescence imaging with biodegradable luminescent porous silicon nanoparticles. Nature Communications. https://doi.org/10.1038/ncomms3326
Further Reading
Last Updated: Oct 15, 2019