Strecker synthesis is one of several means of producing α-amino acids. α-amino acids are molecules, which contain an amine and carboxylic group that are separated by a single carbon (the α-carbon).
Biochemistry scientific background with amino acids. Image Credit: Anna Jurkovska/ Shutterstock
α-amino acids are essential building blocks of proteins, and there are a variety of ways in which they may be synthesized. The Strecker synthesis reaction is lengthy, being comprised of 15 different steps.
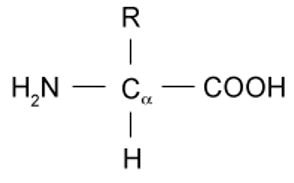
Amino acids possess the general structure as shown below. The R group differs according to the identity of the amino acid. The simplest and smallest amino acid is glycine, for which the R group is a single hydrogen. This is also referred to as an unsubstituted amino acid. R groups that are greater in complexity will result in a substituted amino acid.
Definition
In 1850, the German chemist Adolph Strecker described his reaction:
“A completely different compound was formed upon the bringing together of the aldehyde-ammonia adduct and hydrocyanic acid in the presence of acids.”
In other words, an amino acid is synthesised from the reaction between an aldehyde and ammonia, followed by hydrogen cyanide. Hydrolysis of the resultant product yields the α-amino acid.
Strecker Synthesis of Alpha Amino Acids
Strecker synthesis is simplistic in its requirements. Only three components are required in a two-stage process. This makes it a favourable means of α-amino acid synthesis. Furthermore, the reaction is postulated to mimic the prebiotic process that first produced α-amino acids in earth. As such, it offers a possible method for the synthesis of α-amino acids on primitive Earth. Moreover, this is a researched area that is featured prominently in science with regards to prebiotic, systems and synthetic chemistry.
Overall, Strecker synthesis converts an aldehyde or ketone, and ammonia into an α-amino acid using a metal cyanide, acid catalyst and water.
The two stages of the reaction can be summarised as follows:
- Stage 1: Addition of ammonia and hydrogen cyanide to an aldehyde to form an α-amino nitrile.
- Stage 2: hydrolysis of the α-amino nitrile to produce an α-amino acid.
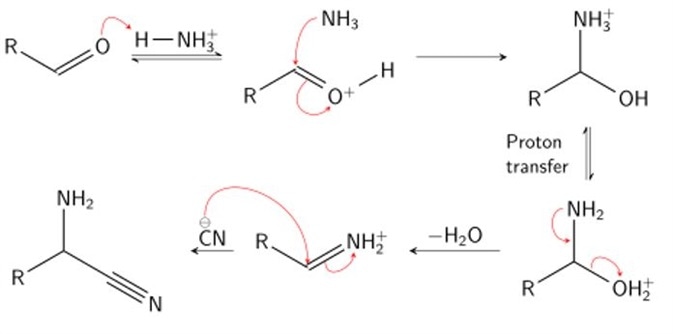
Stage 1
In stage one, condensation of an aldehyde and ammonia occurs. The effect of such a reaction is to protonate (the addition of a proton, H+) the carboxyl oxygen by a hydrogen of the ammonium ion, and add an amine group to the carbonyl carbon by the resultant ammonia molecule. Following proton transfer, water is cleaved from the molecule, producing an iminium ion intermediate.
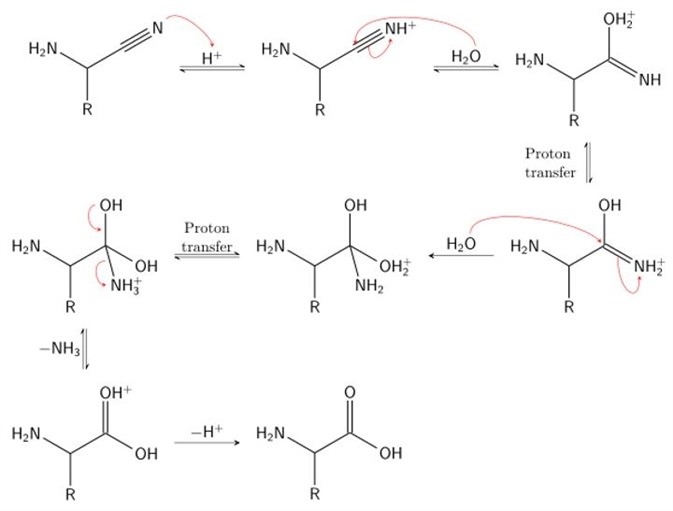
Stage 2
In stage 2, hydrogen cyanide is subsequently used to attack the iminium ion. Cyanide is added across the imine double bond, producing a nitrile intermediate. A nitrile is simply a cyanide group carbon, characterized by a carbon triply bonded to a nitrogen. To do so, the α-amino nitrile is broken down using acid (acid hydrolysis) to produce an α-amino carboxylic acid. Here, the α-amino nitrile is protonated by an acid, undergoes a proton transfer step and is subsequently attacked by a molecule of water. This produces a 1,2-diamino-diol. The acidic conditions of this step result in protonation of the amide and deprotonation of a hydroxyl group. This facilitates displacement of the amine by water. Rearrangement of this product yields the characteristic carboxylic acid group and the final amino acid:
Variations of Strecker Synthesis
The amino acid products can be varied by changing the nature of the starting materials. The use of ammonium salts results in the production of unsubstituted amino acids. Alternatively, the use of primary or secondary amines in place of ammonia results in substituted amino acid. Similarly, the use of ketones in place of aldehydes will produce α-disubstituted amino acids. These amino acids have an alkyl group that replaces the hydrogen at the α position.
Further Reading
Last Updated: Oct 30, 2018