Skip to:
Conventional light microscopy has its limits. Light, being a wave, is subject to diffraction which severely limits the size of structures one can resolve. To be able to observe parts of an organism smaller than this, other techniques need to be employed.
The types of microscopy employed (allowing the user to see such things as molecules and organelles in high detail) fall into two categories: super-resolution microscopy (SRM) and electron microscopy (EM). Both SRM and EM are useful tools for the research, fulfilling different roles in a project.
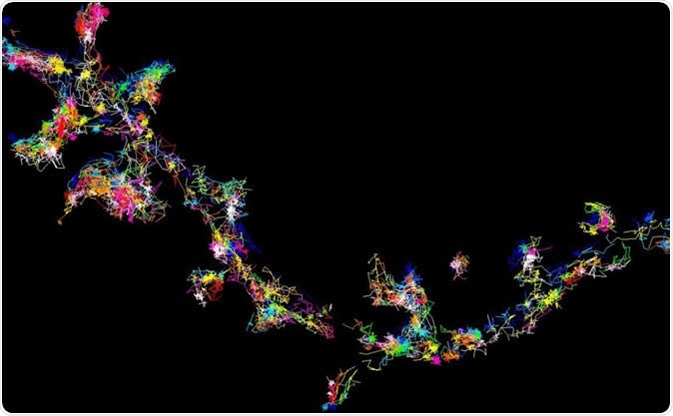
The signalling protein Fyn moving and forming clusters in living brain cells - viewed using super-resolution microscopy. Image Credit: Meunier Lab, University of Queensland
Super-Resolution Microscopy
SRM encompasses a variety of methods. Some of them are listed here, but there are other techniques that can be utilized by a laboratory.
Stochastic optical reconstruction microscopy (STORM)
This is the most commonly used SRM method. STORM uses photoswitchable fluorophores to generate high-resolution images. Specific labelling is then required to achieve a high-quality multicolour image. An optimized STORM system can generate images with a 5 nm resolution, much higher than light microscopy alone.
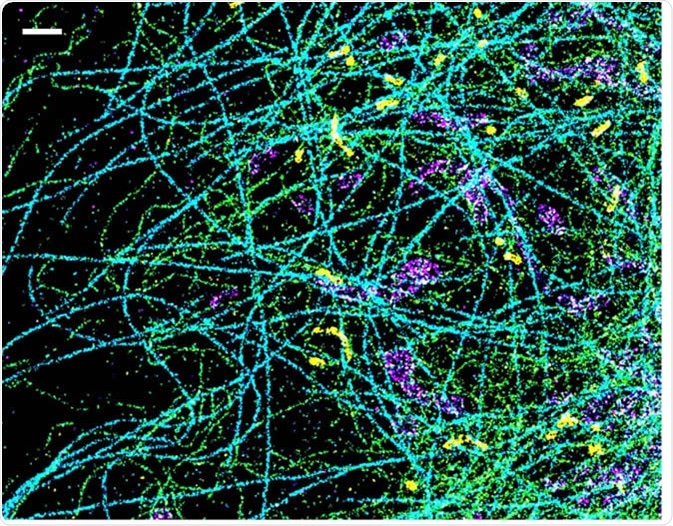
A spectrally resolved super-resolution microscopy image of four subcellular targets that were labeled by four far-red dyes at 10 nm spectral separation. Color is used to indicate the measured fluorescence emission position of each single molecule. (Scale bar: 1 μm). Image Credit: Ke Xu/Berkeley Lab
Structured illumination microscopy (SIM)
This technique involves illuminating samples with patterned light and using specialized software to analyze the information. Resolutions 2-fold higher than the diffraction limit can be obtained by using reconstruction software. SIM’s main advantage over other SRM methods is that it can image thicker sections, which is useful for 3D and live-cell imaging.
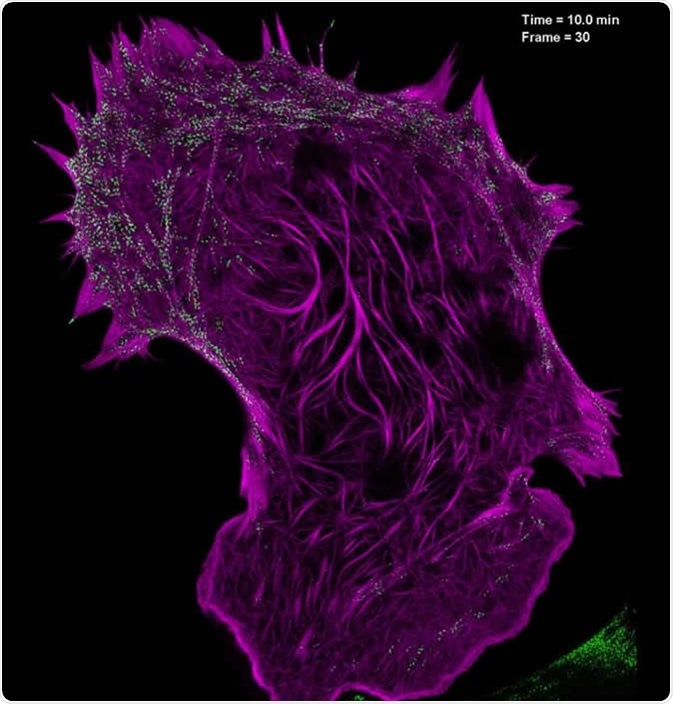
Still image from a video showing the interaction of filamentous actin (mApple-F-tractin, purple) with myosin IIA bipolar head groups (EGFP, myosin IIA, green) at 20-second intervals for 100 time points, as seen with high-NA TIRF-structured illumination microscopy. Image Credit: Betzig Lab, HHMI/Janelia Research Campus
Stimulated emission depletion (STED)
STED is a technique that uses a pair of laser pulses to excite and de-excite fluorophores; the effect is to localize fluorescence at focal spots. Images are then created using raster scanning. Though this technique has a slow acquisition speed for large fields of view, the technique has a large imaging depth of 10-15 µm, allowing images with a high resolution to be produced.
Two-photon excitation (TPE)
TPE’s main advantage is that it causes less phototoxicity than laser scanning microscopy (CLSM) or laser confocal scanning microscopy (LCSM). Unlike in conventional fluorescence microscopy, where the excitation light has a smaller wavelength (i.e. a higher energy) than the fluorescence light, TPE uses usually infrared light at high flux, and fluorescence is observed in the event of a two-photon absorption – at a shorter wavelength than the infrared exciter light, since the combined energy of two exciter photons had been absorbed. TPE has been used in diverse application with live cells, including cytoskeletal organization and reactive oxygen detection.
Though they have obvious advantages over light microscopy for the analysis of cellular structures, the various types of SRM do have their limitations.
Artefacts can still exist in image reconstruction, photobleaching can occur, and the use of specialized equipment and fluorescent dyes can make these processes expensive.
It can take a long time to reconstruct images with some techniques, further adding to the cost of research projects.
Super-resolution microscopy, whilst it can allow the observer to peer more deeply into nature, cannot be used at the molecular level.
For this type of research, there is an even more powerful tool available to scientists that has been around since the early 20th Century: Electron microscopy.
Electron Microscopy
Developed in 1926, electron microscopy works by firing a beam of concentrated electrons at a sample.
The main advantage of this technique is that structures can be analyzed in minute detail, as the resolution of images which are produced using electron microscopy are in the range of down to 0.2 nm, 1000x more detailed than is achieved with conventional light microscopy, and much higher than with SRM techniques.
With EM, scientists can look at nanostructures that would not be visible otherwise, and discern their properties.
There are two main types of electron microscopy:
Transmission electron microscopy (TEM)
Used to view thin specimens (such as tissue sections) by passing electrons through them and creating an image. If a sample absorbs or scatters electrons, the location will appear dark in the TEM image, while if a sample does not interact with the electron beams a bright spot occurs from transmitted electrons. In this way, TEM is analogous to conventional light microscopy, though it uses electrons rather than light and has a far higher resolution.
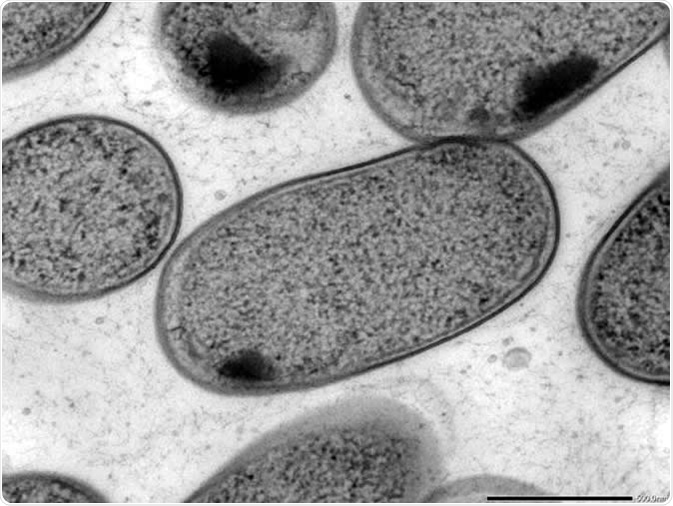
Transmission electron microscopy (TEM) of in vitro-cultured Klebsiella (Kp-2H7). Image Credit: Alessia Ranciaro
Scanning electron microscopy (SEM)
SEM creates images by scanning a surface with a focused electron beam and detecting the backscattered electrons. Contrary to TEM, a location that strongly scatters electrons will appear bright, while samples which do not interact with the electron beam remain dark. This technique provides details of whole cells and surfaces, something which is not achievable with TEM.
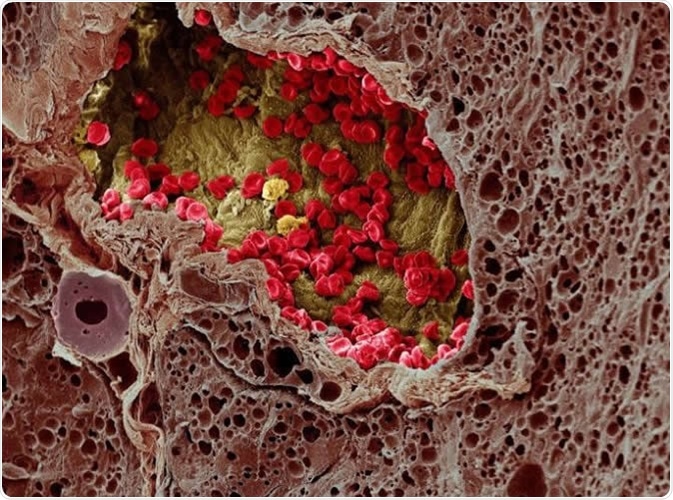
A blood vessel (center) provides essential nutrients to a melanoma tumor (left) in this artificially colorized microscopy image. Image DOI: 10.7295W9CIL38960 Researchers at the University of Tokyo studied a cell membrane receptor, LRP1, and an enzyme, tPA, for their roles in metastasis of melanoma in mice. Image Credit: Wellcome Images CC-BY-NC-ND http://www.cellimagelibrary.org/images/38960
Disadvantages of Electron Microscopy
Electron microscopy, though a powerful tool, does have its drawbacks. The fact that electrons can easily be deflected by molecules in the air means that specimens must be held in a vacuum.
This means that electron microscopy cannot be used to analyze live specimens and structures (whereas certain SRM techniques can), which presents difficulties for biological analysis.
Samples must be preserved in such a way as to represent as closely as possible living structures, which can be costly and time consuming.
Also, the specialized nature of the techniques requires highly skilled operators, and artefacts from sample preparation are common.
SRM vs. EM: Two Powerful Tools for the Researcher
This article has been a brief insight into super-resolution and electron microscopy, outlining their strengths and weaknesses. Both are strong additions to the laboratory and complementary in the information they provide.
Both types of microscopy share that they surpass the resolution limitations of light microscopy.
For life sciences studies, both SRM and EM can resolve cellular structures and thus allow deep insights into the cell function.
While SRM enables scientists to study the cell architecture dynamically in living cells, the resolution is usually lower than with electron microscopy. However, EM requires sample fixation and thus is a method to study cell architectures in non-living samples.
To carry out effective studies, laboratories and their staff should be confident and knowledgeable in the application of these techniques, and up to date on the latest developments in this scientific field.
Who knows what advances the future may hold?
Further Reading
Last Updated: Sep 20, 2019