For a long time there was a controversy about how anesthetics work. The large number of molecules that have anesthetic properties (from benzodiazepines to nitrous oxide) and their very different structures, combined with the results from some experiments, led Meyer and Overton to conclude that anesthetics exert their action by interacting with the lipid membrane of nerves.
However, this theory was proved to be wrong, and it was finally discarded when crystallographic studies demonstrated that anesthetics act by binding to specific active sites of some target proteins.
Nevertheless, although their action goes through the interaction with proteins, not all of them target the same proteins or act in the same way. For example, propofol binds the post-synaptic GABAA receptors and maintain them open, allowing the entrance of Cl- ions. However, benzocaine, a local anesthetic, blocks the voltage-gated Na+ channels.
There is an excellent paper by Nicholas P. Franks (British Journal of Pharmacology (2006) 147, S72–S81) that gives an overview of the history of anesthesia.
Do different anesthetics target different proteins?
Certainly. But even a given anesthetic may target different proteins. For example, propofol is known to target GABAA receptors, but, in addition, recent studies point to its interaction with the CB1 receptors of the endocannabinoid system.
How specific are anesthetics, i.e. do anesthetics target only one type of protein or can they also interact with other proteins?
This is a key point. As it happens with any other drug, the anesthetics have a given affinity for the target proteins, but they also have affinity for some (or in some cases many) other proteins. The anesthetic will preferentially dock into its target proteins, but depending on the dosage, it may attach to other kinds of receptors, yielding undesired effects.
A very well-known case is the accidental death of the Pop Star Michael Jackson, who died from an overdose of propofol, lorazepam and diazepam. The anesthetics may act as respiratory depressants and some may also target beta-adrenoreceptor-mediated signal transduction in cardiomyocytes.
Please could you tell us about how you have combined mass-resolved electronic spectroscopy and ab initio calculations to model the interactions of anesthetics with proteins?
The interaction between anesthetics and proteins takes place through what is called “non-covalent interactions”, such as hydrogen bonds, electrostatic interactions, London forces and dispersive interactions. The strength of such interactions are very small compared with that of a covalent bond, like for example between two carbon atoms or between a carbon and a hydrogen. However, as there are many of such interactions between, for example, a ligand and a receptor protein, they can even modify the shape of the molecules. Actually, the non-covalent interactions are responsible for the tertiary structure of the proteins.
Thus, a deep knowledge of how such forces work is very important to understand the ligand-protein interaction, and therefore to understand the anesthetic-protein interaction. However, as they are weak it is hard to characterize them.
One of the most popular sources of data is the X-ray structures, but forming a crystal usually introduces a significant distortion on the equilibrium distances and geometries of the molecules and therefore such data are not as good as one would expect to model the non-covalent interactions.
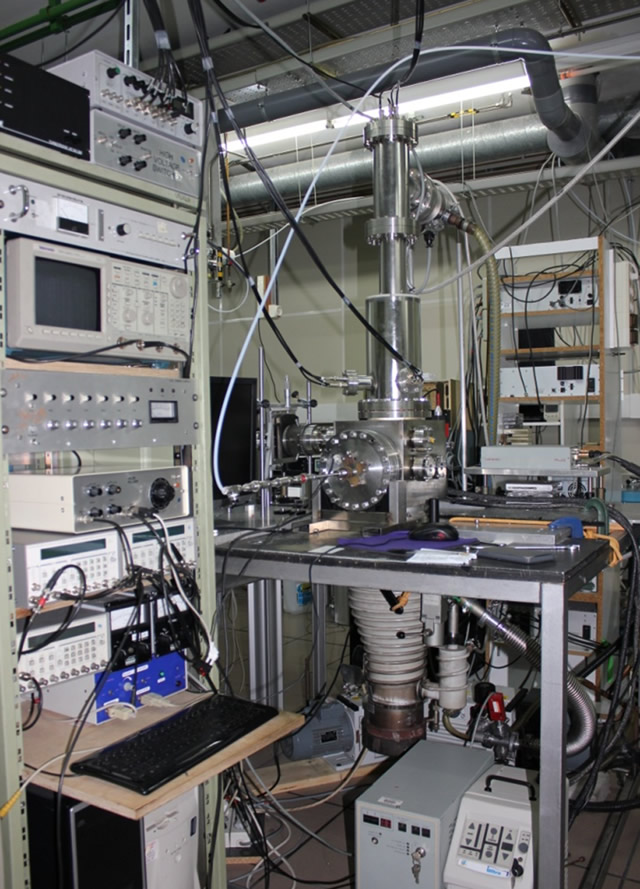
A picture of part of the experimental set up employed in the research.
Another approach consists of dividing the interaction between the protein and the ligand (anesthetic in this case) in ligand-amino acid pairs of interactions. Then, it is possible to put both molecules in gas phase using laser ablation and, using a carrier gas, to create an expansion that cools both molecules to a few Kelvin.
Under such conditions, the molecules first aggregate due to the non-covalent interactions, and after a few microseconds, they fly without interaction with the rest of the species in the beam. In this cold, collisions-free environment the anesthetic-amino acid clusters are free to adopt many possible conformations, each of them corresponding to a different way in which the two molecules can interact, and therefore is the result of a different and subtle balance between all the non-covalent interactions at play.
Now, to extract the structural information from each different conformer, the expansion is created inside the ionization chamber of a time-of-flight mass spectrometer and it is interrogated using different laser pulses and a number of mass-resolved laser-based experimental techniques:
- using REMPI (Resonance enhanced multiphoton ionization) it is possible to obtain the vibrationally-resolved electronic spectrum of the cluster;
- the UV/UV hole burning technique allows determining the number of conformers for each cluster
- the IR/UV double resonance technique allows obtaining the mass-resolved IR spectrum
Such results are compared with the predictions from ab initio calculations. In this way, it is possible to obtain an accurate description of the cluster’s structure, and of the importance and nature of the non-covalent forces at play.
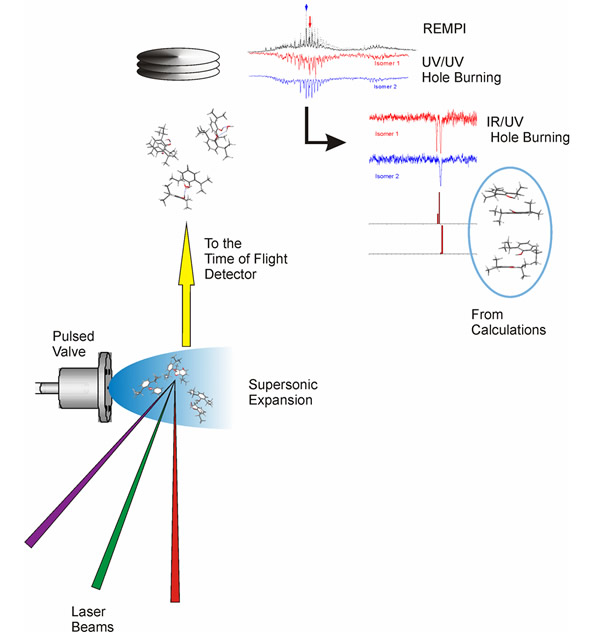
Flow diagram of the experiment. The molecules are introduced in the mass spectrometer using a supersonic expansion, which is interrogated using several laser beams. The spectra obtained contain important structural information, which is interpreted on the light of quantum chemical calculations.
What has your research currently found?
We are engaged in the study of propofol, using the above-described methodology. On the one hand, we have explored the solvation of propofol, determining the structure of propofol/water complexes and of two molecules of propofol with several water molecules. This information is also relevant to solve the problem of propofol solubility. On the other hand, using the X-ray data published on the interactions that take place between propofol and the GABAA receptor, we have characterized the clusters between propofol and the residues of two key amino acids: tyrosine and threonine. Part of the work is still unpublished and there is more going on, to form clusters between propofol and di/tri-peptides, to get even closer to the real system.
What impact do you hope your work will have?
Our data will contribute to understand why propofol attaches to that particular site in the receptor and how are the forces driving such docking process. The whole docking process is governed by changes in Gibbs free energy between propofol solvated by the extracellular medium and by the active site. Thus, it is necessary to characterize both situations to understand the process.
Furthermore, the equilibrium distances and geometries obtained in this work may be used to refine the molecular mechanics computer programs, which would result in a better predictive power of such tools.
How can anesthetics be designed so as to prevent undesired interactions with proteins?
This is a hard question. The ideal drug should only target the desired protein(s). All the rest of the interactions may result in undesired side effects. With the increasing in power of the modern computers we may be able to simulate in the future the interaction between the drug candidates and hundreds or thousands of proteins, but that day is still far away in the horizon. So far, the best way to find more specific drugs is to carry out screening experiments.
Do you have any plans for further research into this area?
Yes. We are slowly increasing the size of the system to approach as much as possible to the true interaction
Where can readers find more information?
They can find our paper here: http://onlinelibrary.wiley.com/doi/10.1002/cphc.201200633/abstract
About Dr José A. Fernández
 Dr José A. Fernández works in the Physical Chemistry Department of the Faculty of Science and Technology, at the University of the Basque Region.
Dr José A. Fernández works in the Physical Chemistry Department of the Faculty of Science and Technology, at the University of the Basque Region.