Jun 7 2016
Tanaka Holdings Co., Ltd. (Head office: Chiyoda-ku, Tokyo; Representative Director & CEO: Akira Tanae) announced today that Tanaka Kikinzoku Kogyo K.K. (Head office: Chiyoda-ku, Tokyo; Representative Director & CEO: Akira Tanae), which operates the Tanaka Precious Metals manufacturing business, has developed the world's first kit able to directly detect the ZIKA virus (ZIKV) in blood. The kit is capable of rapid ZIKV detection in just 10 to 15 minutes. Tanaka Kikinzoku Kogyo plans to supply samples for clinical evaluation with a view to collaboration with domestic and overseas medical manufacturers.
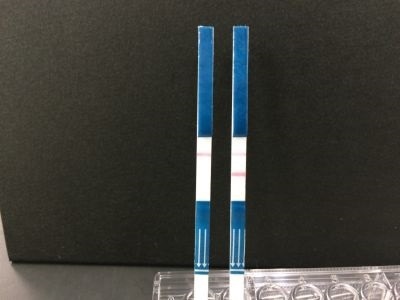
Demonstration of testing (On the left is a negative and on the right a positive reaction. When the virus is detected, two lines appear.)
Characteristics of detection kit
By making it possible for the first time to directly detect ZIKV in blood by immunochromatographic assay, the kit realizes simpler, faster and lower cost detection than existing methods.
Characteristic (1): world's first kit to detect ZIKV via immunochromatographic assay
There is already an immunochromatographic assay method in practical application that detects the antibodies created in the human body following ZIKV infection, but the new kit makes it possible for the first time to directly detect ZIKV itself when present in the blood. By applying its unique antibody screening technology and nano-colloidal gold, Tanaka Kikinzoku Kogyo has developed the new kit using antibodies to nonstructural protein (NS1) in ZIKV, allowing ZIKV to be detected at a concentration of 102TCID50/mL. This detection performance equals the sensitivity of other immunochromatographic assay-based test kits for influenza and other pathogens.
Characteristic (2): simpler, faster and lower in cost than existing methods
The existing method used for detecting ZIKV in blood is PCR, which requires special equipment and takes between half a day and one day to complete. With the new kit, however, the test strip only needs to be dipped into the test sample to enable ZIKV detection. Additionally, it allows detection in 10 to 15 minutes, equaling the simplicity and speed of the influenza virus detection kit already in practical application. Furthermore, unlike PCR, it does not require special equipment, thus realizing cost savings for users.
Background to development of detection kit
ZIKV infection has been spreading since 2015, particularly in Brazil. Infected people develop commonly Zika fever, with symptoms of fever and conjunctival congestion. If pregnant women become infected with ZIKV, there are indications that the fetus may develop microcephaly, which is an abnormal smallness of the head, a congenital condition associated with incomplete brain development. As the possibility has been indicated of infection not only through mosquitoes, but also through blood transfusions and sexual contact, a kit is required which can perform direct and specific detection in the early stage after infection using a fast and simple method. The simple ZIKV detection kit that is currently commercially available detects the antibodies to ZIKV (immunoglobulin G and immunoglobulin M) that are producted in the bodies of infected humans, and is therefore not suited to diagnosing Zika fever in the initial stage of infection. In order to make possible early identification of ZIKV infection, Tanaka Kikinzoku Kogyo applied its store of technology, its unique antibody screening technology, and the nano-colloidal gold manufacturing technologies which it has accumulated over many years to develop a kit capable of direct detection of the virus itself.
It is hoped that the development of the new kit will be highly effective in suppressing the spread of ZIKV infection.
Future rollout
The spread of ZIKV has become a worldwide threat, with the World Health Organization (WHO) declaring on February 1, 2016, that the Zika fever it causes represents a 'public health emergency of international concern'. It is estimated that more than 4 million people are already infected on the South and North American continents. Specifically in Brazil, there is reportedly concern that this summer's Rio Olympics will see a further spread of the infection.