Bone graft substitute comprised of viable, lineage committed bone cells delivers all three properties required for bone formation.
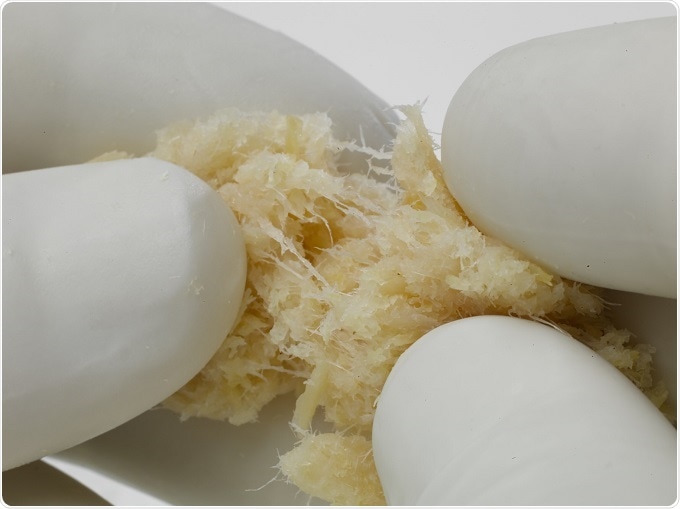 DePuy Synthes*, in collaboration with LifeNet Health®, launches ViviGen Formable™ Cellular Bone Matrix**, a second generation cellular allograft used to assist in the formation of bone during spinal fusion surgery. ViviGen Formable augments the DePuy Synthes biomaterials portfolio and joins the first generation, ViviGen® Cellular Bone Matrix, which launched in late 2014. With both ViviGen and ViviGen Formable, surgeons may now choose a preferred handling option based on the needs of each surgical case.
DePuy Synthes*, in collaboration with LifeNet Health®, launches ViviGen Formable™ Cellular Bone Matrix**, a second generation cellular allograft used to assist in the formation of bone during spinal fusion surgery. ViviGen Formable augments the DePuy Synthes biomaterials portfolio and joins the first generation, ViviGen® Cellular Bone Matrix, which launched in late 2014. With both ViviGen and ViviGen Formable, surgeons may now choose a preferred handling option based on the needs of each surgical case.
ViviGen Formable contains osteoinductive, demineralized fibers that create a putty-like consistency. The fibers interconnect to achieve the formable handling favored in open void cases such as posterolateral fusion, a common type of spinal fusion surgery. This unique handling helps surgeons mold the allograft into a defined shape, allowing ViviGen Formable to conform to the surgical site.
“ViviGen Formable has a great consistency, which allows it to be packed into the openings of the posterolateral spine,” said Dr. Thomas Morrison, MD*** of Polaris Spine and Neurosurgery in Atlanta, Ga. “The handling characteristics of ViviGen Formable deliver confidence that the fusion bed is well packed and grafted for fusion.”
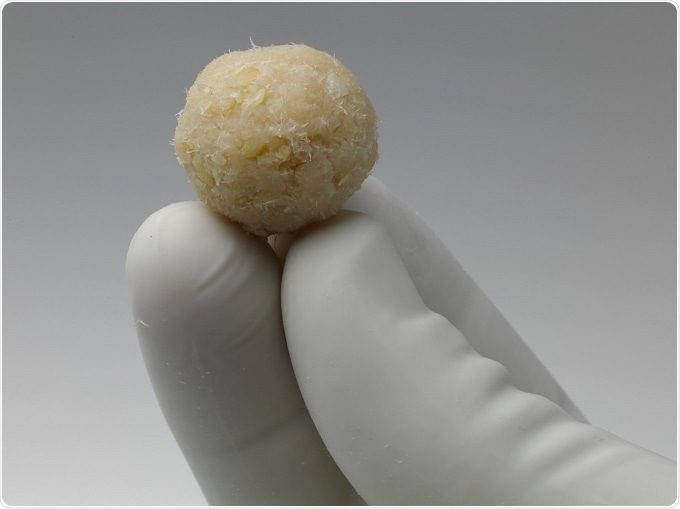
Both ViviGen and ViviGen Formable represent a paradigm shift in the field of bone repair by focusing on protecting viable, lineage committed bone cells from recovery to implantation while retaining all three properties required for bone formation, osteoinductivity, osteoconductivity and osteogenecity. ViviGen is now being used in a wide variety of trauma procedures for the repair or reconstruction of musculoskeletal defects.
ViviGen and ViviGen Formable are Human Cells, Tissues, and Cellular and Tissue-based Products (HCT/Ps) comprised of cryopreserved, viable cells within a cortical cancellous bone matrix and demineralized bone. Over the past several years, LifeNet Health has conducted extensive research to enable the processing of these products to maintain cell viability, resulting in an acceptable alternative to autograft.
DePuy Synthes has an exclusive worldwide agreement to market and promote ViviGen and ViviGen Formable.