In this interview, we speak to Keith Olson and Coby Carlson from FUJIFILM Cellular Dynamics about their iPSC technology and how it is making breakthroughs in blood-brain barrier research.
Please can you introduce yourself and tell us about your role at FUJIFILM Cellular Dynamics?
Coby Carlson: My name is Coby Carlson, and I lead the Applications Team at FUJIFILM Cellular Dynamics. Our team is separate from the R&D scientists, staff, and experts focusing on stem cell culture and differentiations to create unique, specialized cell types. Our role is to test the functionality of these cells, demonstrate their performance on various platform technologies, and develop applications.
Keith Olson: My name is Keith Olson, and I am the Executive Vice President for Commercial Operations at FUJIFILM Cellular Dynamics.
FUJIFILM Cellular Dynamics is a global leader in developing and manufacturing human induced pluripotent stem cells (iPSC). Please can you tell us more about some of your core aims and missions as a company?
Coby Carlson: Fujifilm’s core technologies include reprogramming, engineering, and iPSC culture and differentiation to create unique cell types. Our mission is to bring these technologies to an industrial scale. The technology was first invented over 15 years ago, and since then, we have continuously improved and optimized it to launch it on a large scale. Our goal is to make different cell types accessible all over the world.
Keith Olson: I believe that we were pioneers of iPSC technology in the United States. We successfully brought it to market, commercialized it, and built a business around it. Our reputation is now based on our core expertise, quality, and ability to manufacture at scale for our clients in the pharmaceutical, biotech, and academic sectors.
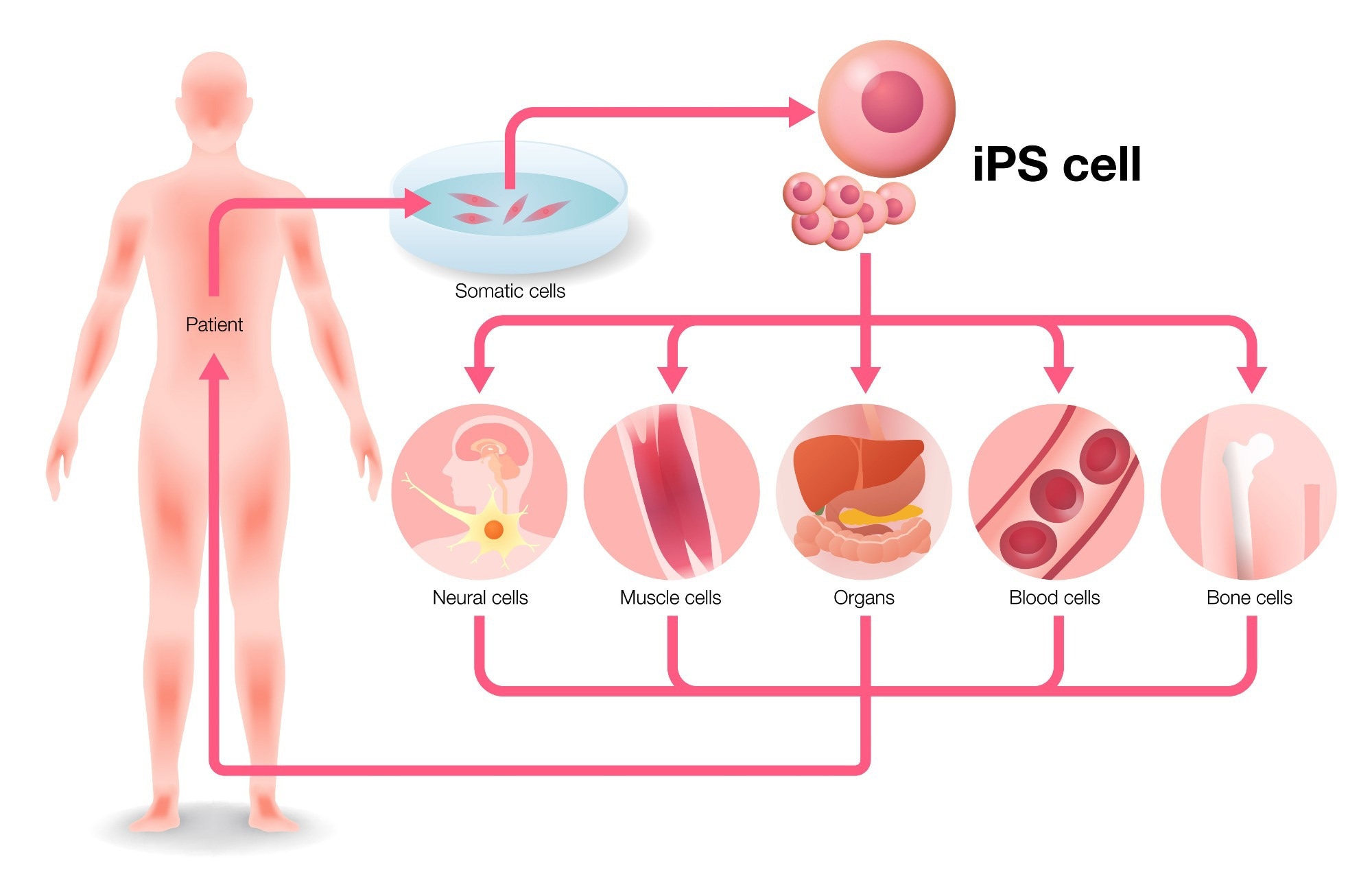
Image Credit: metamorworks/Shutterstock.com
iPSC technology can be used to revolutionize scientific research and cell therapy. Why is this, and in what application areas within scientific research does this technology benefit the most?
Coby Carlson: The primary focus of people working with iPSC technology is toxicology/safety pharm, disease modeling, and cell therapy. Safety is crucial because we can generate cardiomyocytes from iPS cells, and these cells spontaneously beat in a dish while responding to known cardiotoxic molecules.
To measure the safety and effectiveness of drugs in a dish, we use iPSC-derived cardiomyocytes in safety toxicology. We have also participated in the CiPA (Comprehensive in Vitro Proarrhythmia Aassay) initiative to standardize this process across various labs globally. The recent FDA Modernization Act has suggested using alternatives to animal testing, making iPSC-derived cardiomyocytes a crucial component of measuring the safety and effectiveness of drugs going forward.
Keith Olson: iPSC delivers a specific gap in the market by providing customers with high-quality human cells. Instead of researching animal models or transformed cell lines, researchers can now use real human cardiomyocytes or neurons for more biologically relevant results.
At FUJIFILM Cellular Dynamics, you offer a range of iPSC cells, including neural and cardiac cells. Can you tell us more about some of the products you offer and how they are generated?
Coby Carlson: The uniqueness of iPSC technology lies in the fact that there are not many human donors who can offer their brain cells. This technology provides access to different cell types, and we can differentiate highly specialized cells to obtain unique features such as excitatory neurons and microglia. These unique cells are essential for in vitro assays, where we can test them differently than we would in an animal model. For instance, mouse models are different from human models.
Keith Olson: Everything starts from a core iPS cell bank, and we have developed specific differentiation protocols for each terminal phenotype of the cell. Whether it is a cardiomyocyte, neuron, or hepatocyte, we have a differentiation protocol that takes us from the core stem cell bank to a finished product that customers can purchase and use as an assay-ready sample.
Image Credit: Magic mine/Shutterstock.com
The blood-brain barrier (BBB) is vital in understanding how the brain functions, and its dysfunction can lead to neurological conditions such as MS, stroke, and epilepsy. Why did you choose to focus on this area, and how can your iPSC technology help to accelerate breakthroughs?
Coby Carlson: Researchers and drug developers have long struggled with the blood-brain barrier, a critical cell structure separating the blood from the brain. The barrier keeps out harmful pathogens and toxins while allowing vital nutrients to enter the brain. However, it also prevents drugs from crossing over and treating neurological diseases such as neurodegenerative disorders.
The main challenge has been replicating this barrier in vitro. One of the reasons we pursued iPSC cell technology is that we developed ways to distinguish specialized cell types from the same donor to create the blood-brain barrier. Additionally, we can cryopreserve these cells and generate media that allows them to be functional and reanimated from the thaw, enabling us to test them in an in vitro assay.
Keith Olson: We chose to develop a highly relevant human model for studying the blood-brain barrier for several reasons. The industry bottleneck and challenge in understanding how some large molecules cannot pass through the barrier made it necessary. We needed a system that truly replicated what happens in the human brain. We helped revolutionize the market by making the cell type, which was particularly difficult to cryopreserve and develop as an assay-ready product. We were able to deliver a fully isogenic solution with all cell types, and now customers can build their own real human BBB in an assay plate.
At Neuroscience 2022, you showcased research surrounding the establishment of a BBB model using iPSC-derived human cells. Can you tell us more about this research and the potential to integrate this BBB system with emerging organ-on-a-chip technologies to advance the field of drug discovery?
Coby Carlson: Generating the BBB is a significant challenge that requires assembling three different cell types. We validated our model using a Transwell assay, which measures the function of the cells on both the top and bottom of the barrier. However, we recognize that the BBB is a complex system, and microfluidic and organ-on-a-chip technologies offer a unique environment for the cells to function. Combining iPSC technology and OoC technologies will enhance cell function in these systems by providing unique environments with microfluidics and dynamic flow similar to the bloodstream. This will allow us to demonstrate the advantages of these models in a more comprehensive manner.
Keith Olson: The system is perfect for 3D modeling because it is a 3D system. It is a multi-layer, multi-cell-type organ or part of an organ, and using it as an organoid or spheroid in a complex model brings you closer to that model system. Building the barrier is crucial, and 3D modeling is the best way to achieve this. Our released product is flexible enough for clients to conduct assays in a Transwell plate or as a spheroid. They can even use more sophisticated organ-on-a-chip systems to create a comprehensive model for this biology.
What challenges are faced when generating new leads in neurological drug discovery? How can your 3D neural spheroids help to tackle these?
Coby Carlson: When we started the company, we differentiated cells from iPS lines to create highly purified neurons. While they were unique and visually pleasing, we discovered that some of their functionality was lacking. Ten years later, we have developed various products, intending to combine them to create multicellular cultures, not just in 2D systems but also in 3D systems.
Our approach involves mixing different cell types to increase complexity and biological relevance, which we believe will help improve testing and facilitate the development of new therapies and the identification of new compounds.
Keith Olson: In neurobiology, it is well-established that a human system is essential. Animal model systems simply do not replicate human biology, and studying true human biology requires a 3D system. We are not flat figures but 3D beings, and building a real neural system allows us to observe neural connections, communication, and the impact of glia on neurons, studying how cells interact as they would in the body. This is where the true value lies in what we are delivering.

Image Credit: Naumova Marina/Shutterstock.com
Are you hopeful that with continued research surrounding the BBB, we will see new advancements being made surrounding health and disease? What would this mean for both patients suffering from neurological diseases as well as clinical settings?
Coby Carlson: As a technology, the use of human iPS drive cell types is still in its infancy, with significant advances still being made. We believe that some major discoveries will be made by using this technology, whether that be by us or others. This technology has the potential to facilitate the discovery of new treatments, and we are excited about its future potential.
Keith Olson: I think anything that any of our competitors, colleagues, friends, and neighbors are doing in this space will help push the field forward. The more vendors and researchers working on creating relevant model systems for humans, the better. With the FDA Modernization Act and the desire for better test systems and models, everyone working together will likely result in better systems and, hopefully, faster, cheaper, and easier drug discovery for companies.
You were exhibiting at SLAS US 2024, an international exhibition and tradeshow bringing together both world-leading researchers and industry professionals. Can you tell us what you were exhibiting at SLAS and the importance of attending these in-person conferences?
Coby Carlson: The SLAS conference is significant as it brings together various customers, collaborators, friends, and former colleagues operating in this field. The past years have seen challenges due to the pandemic and remote work, with limited ability to collaborate effectively. The opportunity to meet face-to-face is essential when pushing these models forward and persuading companies and groups to invest significant time and resources. Having these conversations in person allows for quick decision-making and improves the efficiency of the process.
Keith Olson: The SLAS conference seems to have finally returned to normality after the pandemic. The number of attendees and vendors is impressive, and the human-to-human interaction is just unbeatable, especially when it comes to answering queries and gaining an understanding of something first-hand. Our focus remains on highlighting our entire portfolio, but we also showcased some new systems, such as the iCell® Macrophage, which is the first of its kind.
Additionally, we continued to promote our BBB system and highlighted other 3D model systems we have developed, including in the neuro and cardio spheres. Overall, it was an opportunity to build awareness for our company and its offerings.
Industrial Scale Cell Engineering - FUJIFILM Cellular Dynamics at SLAS 2023
On your website, you also offer a variety of resources for your customers, including research posters, webinars, and videos. How important are these resources in building customer relationships?
Coby Carlson: Reviews and testimonials are popular among our customers, who are primarily scientists requiring supporting evidence. We understand that content is essential, so we offer it in various formats, including small bites, digital graphical abstracts, and detailed protocols. We aim to cater to the diverse needs of our customers, providing them with the relevant information to make informed decisions about our products.
Keith Olson: We all learned a valuable lesson during the pandemic that content is king. As a result, we had to adapt and utilize various means such as websites, LinkedIn, and other portals to disseminate information to our customers and clients. Due to the absence of trade shows, these platforms were our only options. However, now that trade shows are seemingly back in full swing and everyone is attending, it presents a more effective way to communicate to a large audience at once, which is always more enjoyable.
With so many breakthroughs and technological advancements being made within the life sciences, what are you personally looking forward to the most in the coming years?
Coby Carlson: While we did not discuss it much, our cell therapy is where the truly exciting developments will take place and capture the general public’s attention. For instance, curing blindness by using retinal cells is something that could happen in our lifetime. Although I cannot speculate on the exact timeline, I believe it is inevitable. Our in vitro work behind the scenes in drug discovery and other aspects aligns well with this goal, as the same cells used in our research could be used as drugs in the future.
Keith Olson: An exciting prospect on the horizon is the potential replacement of animal model systems with validated human models within the next year or five years. If this were to happen, and the FDA, pharma companies, and biotechs were on board, it would be a major advancement for everyone. This could result in faster, cheaper, and more efficient processes. One of the main challenges in this field is the high rate of failure, and any improvements made in this area could potentially save billions of dollars for the industry.
What is next for FUJIFILM Cellular Dynamics? Are you involved in any exciting upcoming projects?
Coby Carlson: We always strive to advance our field, evaluating new technologies and creating new products. We want to expand our collaborations and work with others to achieve our scientific goals. While we can conduct R&D, we recognize that partnering with other experts, sharing information, and collaborating can lead to even greater success.
Keith Olson: Our company is constantly exploring new cell backgrounds, and we are committed to launching two to three new cell types each year. Currently, we are focused on introducing three new cell types, all of which will be launched by June or July of this year. Simultaneously, we have begun developing the next three cell types for 2024. Our goal is to continue expanding our offerings, and we hope to return next year with news of three entirely new cell lines.
About Dr. Keith R. Olson
Keith Olson is the senior commercial executive for FUJIFILM Cellular Dynamics, where we have developed the market-leading portfolio of differentiated human cells derived from iPSC.
Keith has previously served in leadership roles for Life Science products at Corning Inc, Life Technologies, DiscoveRx and Cellomics, and has launched over 700 Life Science products in his career. Keith received his bachelor’s degree in Molecular Cell Biology from Carnegie-Mellon University and his Ph.D. in the same from the University of Rochester.
About Dr. Coby Carlson
Coby Carlson is the Director of Applications Development at FUJIFILM Cellular Dynamics where his team focuses on advancing the use of human iPSC-derived cell types for drug screening, toxicity testing, and disease modeling.
He joined the company in 2012 and has extensive experience developing applications with iCell products, characterizing their functional performance on various technology platforms, and building co-culture systems in both 2D and 3D format. Coby received his PhD from the Univ. of Utah followed by a postdoctoral fellowship at UW-Madison.