Nanoparticles (NPs) have become ubiquitous nowadays and are increasingly being used in many different products. A reference material is required whenever nanoparticles of interest are investigated for chemical and physical properties, toxicity, environmental considerations, pharmaceutical prospects, or for any other application.
Access to defined nanoparticle standards is very important for users dealing with queries related to sizing or tracing of nanoparticles, and such standards are often employed for providing the basis for technologies, applications, or evaluation and standardization techniques.
Various organizations, including BBI Solutions and National Institute of Standards and Technology (NIST), offer different qualities of engineered nanoparticles that have defined properties, such as size, shape, and composition.
Producing monodisperse nanoparticles continues to be a well-guarded secret and demands for more monodisperse products require new methods and technologies. This can be achieved by creating well defined procedures for selective isolation techniques or well-defined synthetic procedures.
This article shows how a selective separation technique could be used to acquire pure samples of nanoparticles and how it can be effectively used to integrate both strategies to further boost the monodispersity of nanoparticles, thereby acquiring only pure nanoparticles with user-defined properties.
Monodispersity of Nanoparticles/Nanoparticle Standards
In addition to mass and shape, size-based monodispersity is considered as one of the most critical factors for characterizing nanoparticles of a specified composition. Based on this factor, methodologies are validated, observed effects or results are track-backed, or reasonable conclusions are drawn.
To put it briefly, the term “dispersity” refers to a measure of the heterogeneity of particles or molecules in a mixture. Therefore, the term “monodispersity” refers to a sample (collection of objects) that has constant and similar mass, size, or shape.
Using particle-size distribution (PSD), the relative amount of particles in the sample is defined based on mass, size or shape.
Data processing laser diffraction techniques, with recent advances, automation, and precision, have become the most sought-after technology for determining size distributions. Light scattering (LS) technology allows continuous monitoring and collection of data, which makes it a suitable option for integrating separation and analysis within a single run.
This can be achieved by combining field-flow fractionation (FFF), either asymmetric flow FFF (AF4) or centrifugal FFF (CF3) as the separation method in tandem with appropriate LS detectors. Dynamic light scattering (DLS) can also be used, in addition to static/multiangle light scattering (SLS, MALS) techniques.
This article describes the following factors:
- The potential to fractionate in preparative scale or milligram scale
- The size-distribution analysis of a couple of monodisperse gold nanoparticle standards through AF4 separation
- The option to additionally increase the monodispersity of nanoparticles
- The cleaning and purification of the nanoparticles
- Complete verification and quality check through online AF4-DLS coupling, number-based size distributions (number PSD), and TEM measurements
Gold-NPs or Au-NPs are used as key reference and standardization materials owing to their inertness, stability, and biocompatibility, and these are used as examples for the experiments.
Experimental Setup
In this experiment, an AF4 unit is coupled online to a DLS detector (Malvern Zetasizer Nano® S) and a UV detector for separation and analysis purposes. Real-time sample analysis during the fractionation process is facilitated by the “flow-mode” option, where a unique quartz flow-cell is connected inline to the detector flow stream.
This set up allows the experiment to be continuously monitored. Next, two Au-NP samples of 15 and 40nm size were first examined on the analytical purpose AF4-channel and then on the preparative channel to acquire adequate amounts of nanomaterial.
TEM measurements of the fractionated specimens were used for quality control and validation. This approach is briefly addressed: A continuous linear cross flow is first used. The first ‘injection’ step allows sufficient amount of time to transfer the sample into the channel and then relax during ‘focussation’.
Following the ‘transition time’, the sample elutes and is isolated based on hydrodynamic radius within steady cross-flow conditions.
Preparative Channel and Sample Fractioning
As soon as evaluation is completed in the analytical channel, the method is transferred for the preparative fractionation without making any additional changes. It was observed that FFF took place comparable to the results acquired in the analytical channel. In order to obtain size data about the Au-NPs, the Malvern Zetasizer Nano® S was used in flow mode and directly coupled to the AF2000 unit (Figure 1).
Figure 1. Semiprep AF2000 Channel
Classical Characterization and Preparative Separation Technologies
Preparative Separation
Chromatographic methods are extensively employed for functioning in the preparative scale. While tasks like sample concentration, cartridge loading, and analysis time hold significant interest, they prove to be quite complicated when separating a large amount of material.
In the case of diluted samples, high-pressure liquid chromatography (HPLC) or size exclusion chromatography (SEC) for polymers or particles can be used at the molecular level.
In order to separate more amounts of material for analytical purpose, cartridges (larger) and columns have to be changed and should be accompanied by a resolution loss.
SEC is highly sensitive to shear degradation and thus, reduces aggregation and (un-)specific adsorption effects induced by stationary phase interactions functioning in both preparative and analytical scales.
In this case, the FFF-technology provides the most sensitive means for sample analysis, given that a flow stream easily transports the polymers, nanoparticles, and proteins without any added necessity for a stationary phase.
For preparative AF4, the injected amount for a single run was found to be 0.4mg and 0.2mg for 15nm and 40nm Au-NPs, respectively. Using this technology, users can separate even very small amounts of materials based on the sample.
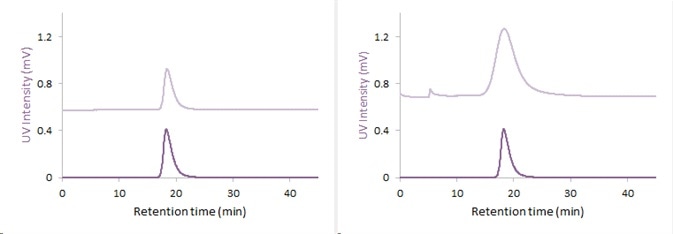
Figure 2. UV-Fractorgam: UV-Fractogram after separation of the 15nm (left) and 40nm (right) gold nanoparticles with AF2000 (blue: 530nm, red: 280nm)
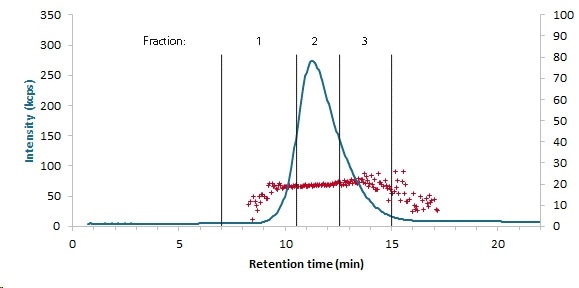
Figure 3. Fractions 15nm: DLS fractogram after preparative AF4 separation (blue: intensity weighted at 173°, red: particle size) and cut-off for the sample fractions of the 15nm Au-NP sample (Dh,av. = 20 ± 2nm).
Particle Sizing
Electron optical techniques are traditionally used for investigating narrow nanoparticle standards. Transmission electron microscopy (TEM) or scanning electron microscopy (STM) can be used based on the particle size. Another useful method is sizing by laser diffraction, which is based on “Mie-scattering” and “Rayleigh” principles depending on the size of particles.
Conversely, the final detected particle size is affected by the technology used and the mathematical and physical techniques applied. One can report particle sizes as geometric (e.g REM and TEM); hydrodynamic (e.g. DLS); or gyration-based (e.g. MALS and SLS) in either diameter or radius.
With the use of mathematical models, certain values can be changed and compared, but users would still need to have a thorough knowledge and study the quantified values.
Conclusion
With the aid of the preparative AF4 setup, users can easily separate even milligram-scale nanoparticles. Monodisperse Au-NP standards (2 sets of samples, 15 and 40nm) that were separated in fractions (each #3) can be obtained and purified, and their monodispersity can be further increased.
For every sample, the cut-off can be tuned to meet the needs of users and thus, produce only those nanomaterials that have defined properties. For isolation purpose, two key improvements can be obtained with the preparative AF4.
AF4 enables an easy and controlled elution of samples, followed by suitable cleaning and purification of samples. This option allows collecting the sample as a whole, and users no longer have to collect specific fractions.
“Real” fractioning of the sample into a series of user-defined fractions is another option used for producing different size of nanomaterials based on the separation in AF4.
As a result, the matching nano-sized samples will be produced by multiple injections in a “truly” milligram scale. At the outset, TEM images of the nanoparticles were obtained for all fractions, results were verified, and distributions were provided.
Acknowledgements
Produced from materials originally authored by Markus J. Spallek, Rainer Jünger, Evelin Moldenhauer, Anna Wallner, Marianne Hanzlik from Postnova.
About Postnova Analytics

Postnova Analytics' mission is to solve analytical problems in biotechnology, polymer and particle science.
Advanced Analytical Products
- Postnova always and only offers high quality products
- Postnova offers the newest and most innovative technologies available
- Postnova only chooses leading manufacturers as supply partner
Powerful Product Portfolio
- Postnova has formed a complete product portfolio (systems, supplies, services)
- Postnova has focused only Field-Flow Fractionation and Light Scattering
- Postnova offers complete solutions from one source with high expertise
Strong Customer Support
- Postnova has a highly qualified customer support team
- Postnova is always looking for long term partnerships with our customers
- Postnova provides the ultimate solution to make customers successful: Quality & Support
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.