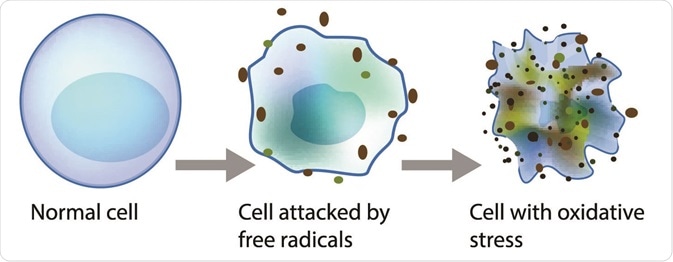
Lipid peroxidation is a metabolic process that causes oxidative deterioration of lipids by reactive oxygen species. This process can degrade the lipids within the cell membrane leading to cell damage and eventually, cell death.
Credit: Pavel Chagochkin/Shutterstock.com
Oxidative deterioration is caused by highly reactive free radical species. A free radical chain reaction occurs during lipid peroxidation as once a free radical is produced, it can react with another stable species to produce another free radical. The products of lipid peroxidation are useful indicators of oxidative stress in tissues and have been linked to the progression of cancer.
Mechanism of lipid peroxidation
The lipid peroxidation process has three steps that form a free radical chain reaction:
- Initiation
- Propagation
- Termination
The initiation step involves production of a fatty acid radical when a reactive oxygen species, such as hydroxyl radical combines with a hydrogen atom to form water and a fatty acid radical. The unstable fatty acid radical quickly reacts with molecular oxygen in the propagation step to form a peroxyl-fatty acid radical.
This unstable radical further reacts with a free fatty acid to produce hydrogen peroxide or cyclic peroxide and another fatty acid radical. The chain reaction of free radical reactions continues until a non-radical species is formed by combination of two free radicals in the termination step. The free radical reactions can also be halted by antioxidant molecules within an organism. They can bind to the free radicals and prevent lipid peroxidation, often in the form of lipid-soluble vitamins.
Products of lipid peroxidation
Lipid peroxidation forms a number of oxidation products, including lipid hydroperoxides (LOOH) and aldehydes such as malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE). LOOH are the primary products of lipid peroxidation produced in the propagation step. After formation, LOOH can be reduced leading to inhibition of peroxidative damage or peroxidative damage induction.
Two electron reductions cause hydroperoxide decomposition that inhibits peroxidative damage, while one electron reduction induces new lipid hydroperoxides by contributing to the initiation and propagation steps. Studies have found that LOOH in serum can be used as an indicator of oxidative stress in tissues.
MDA is the most mutagenic product of lipid peroxidation and is commonly used as a biomarker for oxidative deterioration in omega-3 and omega-6 fatty acids. The thiobarbituric acid (TBA) test uses the reactivity of TBA towards MDA to measure autoxidative degradation within edible fats and oils.
4-HNE is the most toxic secondary product of lipid peroxidation, and it displays a dual role as a protective signaling molecule during gene expression and a cytotoxic promoter of pathological pathways.
Credit: Sakurra/Shutterstock.com
Lipid peroxidation in cancer progression and therapy
Cancer cells have increased levels of reactive oxygen species, indicating a connection between oxidative damage and abnormal cell growth. The amount of lipid peroxidation varies within cancer cells with different concentrations of 4-HNE can be found depending on the origin of tumor.
Studies have found a link between the addition of 4-HNE to cancer cells and a reduction in cell proliferation. Human leukemic cells, that ordinarily have undetectable amounts of lipid peroxidation, strongly decrease cell proliferation when treated with low concentration of 4-HNE. Inhibition of cancer cell growth has also been documented in other tumor types, including colon and breast cancer.
Another study that compared the reaction of human lymphatic leukemia cells and normal human peripheral blood lymphocytes to 4-HNE found a cytotoxic effect only in the lymphatic leukemia cells. 4-HNE is therefore a potential agent to improve therapeutic outcomes of cancer. This could be achieved by adaptations to intrinsic oxidative stress which potentially enhance conventional chemotherapy or radiotherapy which is currently limited by resistance of some cells to apoptosis.
Reviewed by P. Surat, PhD
Sources:
- Vasilaki, A.T. & McMillan, D.C. 2011 Lipid Peroxidation, In Schwab (eds) Encyclopedia of Cancer, Springer, pp. 184-345.
- Ayala, A. et al. 2014. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal, Oxidative Medicine and Cellular Longevity, 2014, e360438.
- Pizzimenti, S. et al. 2010. The "Two-Faced" Effects of Reactive Oxygen Species and the Lipid Peroxidation Product 4-Hydroxynonenal in the Hallmarks of Cancer, Cancers, 2, pp. 338-363.
- Barrera, G. 2012. Oxidative Stress and Lipid Peroxidation Products in Cancer Progression and Therapy, International Scholarly Research Notices Oncology, 2012, e137289.
Further Reading
Last Updated: Apr 16, 2019