Titan Enterprises (Sherborne, UK) reports on its involvement in the development of a unique disposable flow sensor for CRISI Medical Systems (San Diego, USA), a subsidiary of Becton, Dickinson and Company (BD).
Titan Enterprises assisted CRISI in the early days of development of the unique flow sensor used in the BD Intelliport™, a medication management system for manual intravenous (IV) bolus injections. After testing an Atrato ultrasonic flowmeter - CRISI took the basic design, and with help from Titan, miniaturized the flowmeter assembly and made it into a disposable unit. Titan has granted exclusive global rights to BD for use of their Atrato technology in IV bolus injections.
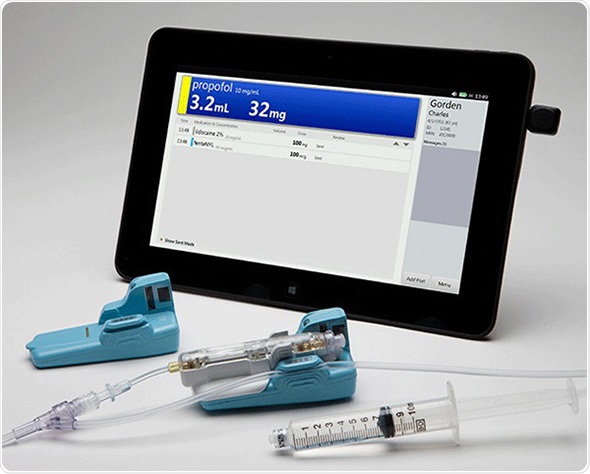
BD and CRISI entered into an exclusive partnership in June 2013 to jointly develop the BD Intelliport™ Medication Management System, a first-of-its-kind medication management solution for manual IV bolus injections. The Intelliport system is the first and only solution to provide real-time drug identification, dose measurement and allergy detection at the point of injection while wirelessly sending captured information directly into the patient’s electronic medical record (EMR) following medication administration. The Intelliport system received clearance from the U.S. Food and Drug Administration (FDA) in December 2014 and is expected to be commercially available in spring 2015.
Titan Enterprises Atrato ultrasonic flowmeter is a true inline non-invasive flow meter without the contorted flow path and disadvantages of alternative designs. It can handle flows from laminar to turbulent and is therefore largely immune from viscosity. It also offers excellent turndown, linearity and repeatability. Available in 60°C and 110°C temperature versions and a 30 bar higher pressure model - Atrato flowmeters use patented 'time-of-flight' ultrasonic technology that enables it to operate over very wide flow ranges (200:1) with excellent accuracy (better than ±1.0% of reading).
With over 40 years’ experience in flowmeter innovation and manufacture, Titan Enterprises philosophy of "pushing the envelope by trying to do things a little different and better" has resulted in sales of over 250,000 products into 40 countries worldwide and a repeat purchase rate of 95%. Today Titan supplies innovative flow measurement solutions to a broad range of market sectors, including medical, chemical, petrochemical, food and drink, laboratory and pharmaceutical. For further information on flow sensors for medical applications please contact Titan Enterprises on +44-1935-812790 / [email protected].