A new statement by the American College of Medical Genetics and Genomics (ACMG), published in the journal Genetics in Medicine, discusses the place of germline genetic testing in breast cancer patients. Clearly, it says, BRCA1/2 testing should not be recommended for all these patients alike, but genetic testing does have an important role which must be exploited by harmonious work between multiple medical scientists researching this area.
Genetic risk in cancer testing
Breast cancer is the female cancer that has the highest number of new cases in the US among all communities and races. It is thought that about 5% to 10% of these are the result of hereditary causes such as disease-causing mutations in the BRCA1 or BRCA2 genes, and to a smaller extent, in other genes like PALB2, PTEN, CDH1 and ATM.
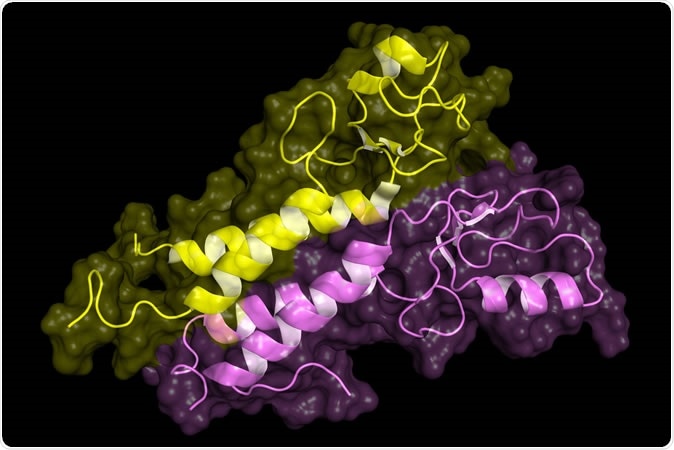
BRCA1 tumor supressor protein RING domain, in complex with BARD1 protein. BRCA1 mutations are implicated in hereditary breast, ovarian and prostate cancer. Image Credit: Petarg / Shutterstock
Knowing if they have a risk of cancer due to their genes allows people to make a choice to go in for early screening and to take preventive action. Again, if the results of genetic testing are available to healthcare providers, it can help take better decisions as to treatment so as to improve the eventual outcome for the patient. However, the crucial point is that genetic testing alone cannot lead to a good prognosis.
Instead, say the scientists, testing must be followed by giving the patient concerned the right kind of care. For this to happen, there must be adequate evidence that one type of care actually works better than another, so that clinical practice guidelines can be set.
The need for the statement
The present document presented by the ACMG is intended to provide a foundation for the current guidelines that are based on existing evidence, by which patients are classified into risk groups in order to make decisions on testing. This helps to codify current practices even while accepting that new information on wider testing plans is still entering the picture.
The document also reveals the unique qualification of and the role of medical geneticists in giving patients the best care, and in helping to determine what are the best practices to deal with patients who already have cancer or have an increased risk of cancer. The ACMG agrees that medical organizations find it difficult to issue guidelines in the area of cancer care and cancer genetics, which are highly complex. Nonetheless, the services of medical geneticists are invaluable in making sense of the evidence available in literature, so that these organizations can create better guidelines. This is especially true with respect to carrying out genetic testing for cancer, because in this case the procedures are best done under the guidance of this type of professional with experience in cancer.
Points to ponder
Some points to consider when testing breast cancer patients for genetic risk include:
- The need to test all patients with breast cancer - it should be modulated by patient characteristics such as the age at diagnosis, the history of family cancer, the presence of estrogen and progesterone receptors, HER2 receptors, and disease stage
- Testing for genetic risk, especially with multi-gene panels, is not based on evidence
- If a pathogenic gene or one likely to be pathogenic is identified in a patient but the gene is not always expressed in all generations where it is present, the patient should be managed with the following things in mind:
- The guidelines here are not evidence-based but rather based on what most clinicians feel is good practice
- More screening has not yet been linked to earlier diagnosis or improved outcomes
- If testing is carried out it should include a full sequencing of the gene, deletion/duplication analysis, and detection of known intronic variants of pathogenic or likely to be pathogenic genes, and the lab should be one that is certified for genetic testing by the Clinical Laboratory Improvement Amendments (CLIA) and/or College of American Pathologists (CAP)
- At the time testing is ordered the patients should be told about its implications and genetic counseling must be given, using the services of professionals who are specialized in cancer genetics
- If a pathogenic or likely to be pathogenic mutation is found associated with breast cancer, and if there are evidence-based guidelines, the benefits of family member testing in cascading manner should be communicated clearly.
Today, medicine is moving towards personalization, with researchers working on the ability to give individual patients the unique care that is suited to their metabolism, their genetic makeup and their medical conditions, among other factors. One important point in achieving this is to make sure patients get the right information so that they can reduce their risk and get the right kind of genomic testing.
Conclusion
In conclusion, the document clearly states, “Currently, there is insufficient evidence to recommend genetic testing for BRCA1/2 alone or in combination with multi-gene panels for all breast cancer patients.” At the same time, cooperation between various medical and related disciplines will help evolve proper recommendations based on evidence, make it easier to get the right care, and make it possible for all people who need to be tested for genetic cancer risk to get it, irrespective of financial, ethnic or racial status.
Journal reference:
Pal, T., Agnese, D., Daly, M. et al. Points to consider: is there evidence to support BRCA1/2 and other inherited breast cancer genetic testing for all breast cancer patients? A statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med (2019) doi:10.1038/s41436-019-0712-x, https://www.nature.com/articles/s41436-019-0712-x