The passive transfer of antibodies is a method of imparting immunity to patients who are infected and ill before their immune system can produce active immunity itself. This approach was used in the 1920s and 1930s, to treat respiratory infections, including the 1918 influenza epidemic. In the context of the current COVID-19 pandemic, one method to passively transfer antibodies has been the use of convalescent plasma (CP).
.jpg)
Effect of Convalescent Plasma on Mortality among Hospitalized Patients with COVID-19: Initial Three-Month Experience. Image Credit: Pirke / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Mortality Following CP Transfusion
Prior research showed that using CP earlier to treat ill patients was associated with a lower mortality rate. Based on this, the researchers looked at the mortality after 7 and 30 days in a large group of over 35,000 adult COVID-19 patients who were hospitalized and transfused with CP. They attempted to detect an association between early treatment with CP and lower mortality compared to later CP administration. Also, they wanted to know if higher antibody levels in CP reduced mortality more than CP with lower antibody titers.
All patients in this study were 18 years or more and hospitalized with confirmed COVID-19. All were severely or critically ill, or physicians in charge of their care determined that they were at high risk of progressing to severe or critical illness.
The findings were validated using two methods of analysis to adjust for confounding factors. The study was ‘randomized’ by the fact that the data came from over 1,800 treatment sites, where the patients were given plasma at variable times from diagnosis, with different titers of antibody in the plasma being given to the patients.
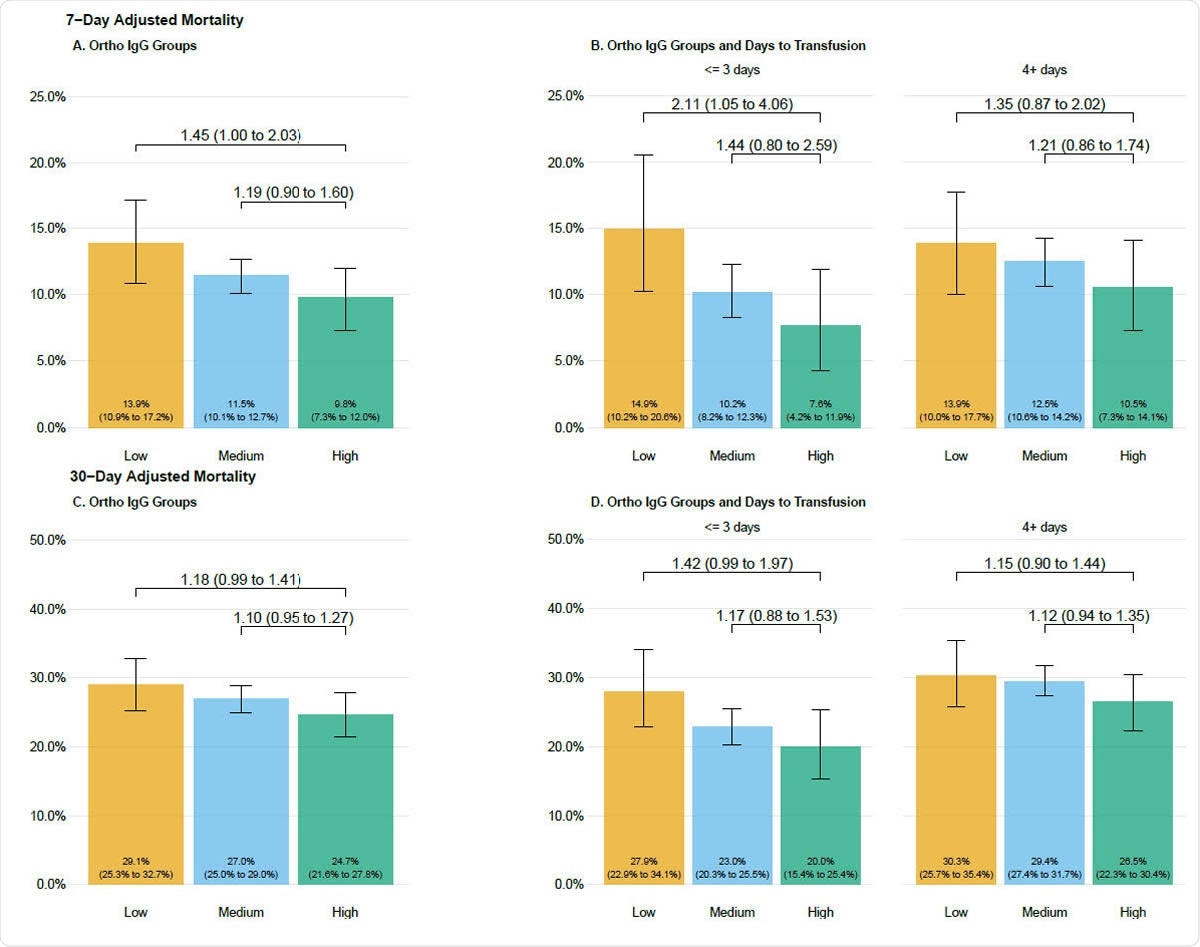
Seven day (A, B) and 30-day (C, D) adjusted mortality stratified by antibody groupings in patients transfused with COVID-19 convalescent plasma. Adjusted mortality rate is presented on the vertical axis, and the height of each bar graph represents adjusted mortality with 95% confidence interval denoted. Data are stratified by groupings of antibody levels with semiquantitative groupings of low (<4.62 S/Co, orange bars), medium (4.62 to 18.45 S/Co, blue bars) and high (> 18.45 S/Co, green bars). Values presented as text within the boxes are the estimated adjusted mortality rates. Values connecting various categories shown with the overbraces are bootstrapped estimates of relative risk and 95% bootstrap confidence intervals. Refer to the methods for the variables in the adjustment and the calculation of the relative risks.
Timeline for Transfusion Benefits
The cohort was classified based on the duration from COVID-19 diagnosis to plasma transfusion, into the following categories: 0, 1-3, 4-10, and 11 or more days. This matches historical data showing that antibody treatment for a respiratory infection is most effective if given within 3 days of hospital admission, rather than later on when the patient is sicker.
This led to the use of a similar criterion for assessment of accuracy but substituting the date of diagnosis for hospitalization.
The results showed that the crude mortality rate fell, at both the 7-day and the 30-day follow-up. Moreover, with the increased availability of CP, the time from diagnosis to transfusion of CP saw a sharp decline. And when patients received plasma within 3 days, the crude 7-day mortality fell compared to those who were transfused 4 or more days after diagnosis, at less than 9% compared to 12%.
Thus, overall, the study suggests the efficacy of CP in a broad sample of patients and treatment facilities in various parts of the USA. The earlier use of CP reduced mortality rates at both the 7-day and 30-day marks.
Another interesting finding is that patients who survived at 7 and 30 days had higher transfused plasma volumes compared to others. This is despite the fact that the investigators had no predefined knowledge of how much plasma would constitute an effective dose before the study began.
Antibody Assessment
The results are similar when the CP has a higher antibody titer – both 7-day and 30-day mortality was reduced. This strongly supports the hypothesis that CP works because of the antibodies in it – in other words, the active ingredient in CP is the neutralizing antibodies against SARS-CoV-2.
At 30 days, the mortality was 30% in patients who received plasma with low antibody titer at 4 or more days from diagnosis, vs. 20% in those who received high-antibody plasma at 3 or fewer days.
This part of the study is limited by the fact that only those plasma units could be assessed where there was a remnant of the sample adequate for assay purposes. The handling and storage of the specimen might, therefore, have impacted the accuracy of measurement of the antibody level. Secondly, the most significant benefit in survival rates was seen when the patients were less sick and were treated earlier. The researchers say more research is required to understand the various ways in which antibodies affect the illness and to identify other factors in plasma, which may change the course of the illness. Such knowledge would help to characterize how plasma helps to counter a viral illness.
Despite very high antibody levels in some units of plasma, there was no evidence of poorer outcomes or a higher death rate in these patients, ruling out the occurrence of antibody-dependent enhancement of disease.
Implications
All the data support the conclusion that the administration of CP with a high antibody titer and given in a timely manner to hospitalized COVID-19 patients will reduce mortality in this group.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources