Most of the vaccine research in the current coronavirus disease 2019 (COVID-19) pandemic has focused on the role of the angiotensin-converting enzyme 2 (ACE2) receptor in mediating viral entry into the host cell. However, a recent study published on the preprint server bioRxiv* in November 2020 uncovers the major role played by the N-terminal domain of the SARS-CoV-2 virus in host infection. This could be of great use in measuring the usefulness of antibodies used in convalescent plasma therapy for COVID-19.
However, the RBD is structurally linked to the N-terminal domain (NTD) of the adjacent spike protomer, and monoclonal antibodies against the NTD neutralize infection, suggesting that the “NTD, as well as RBD, play a pivotal role in SARS-CoV-2 infection.”
Study: The N-terminal domain of spike glycoprotein mediates SARS-CoV-2 infection by associating with L-SIGN and DC-SIGN. Image Credit: NIAID

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Supporting evidence for the role of the NTD
Though COVID-19 is primarily a respiratory infection, with the major complication in severe cases being pneumonia, ACE2 is not highly expressed in lung cells, occurring at relatively low levels in type II pneumonocytes. This has caused understandable confusion, added to which recent findings have shown that non-ACE2-expressing cells also contain SARS-CoV-2 RNA. Thus, there may well be other receptors that allow the virus to gain entry into specific organs like the lungs.
The researchers from Osaka University, Japan, used a lung cDNA library to identify potential receptors, as well as the mechanism by which the virus successfully spreads to other organs such as the intestines, liver, kidneys and blood vessels in infected patients.
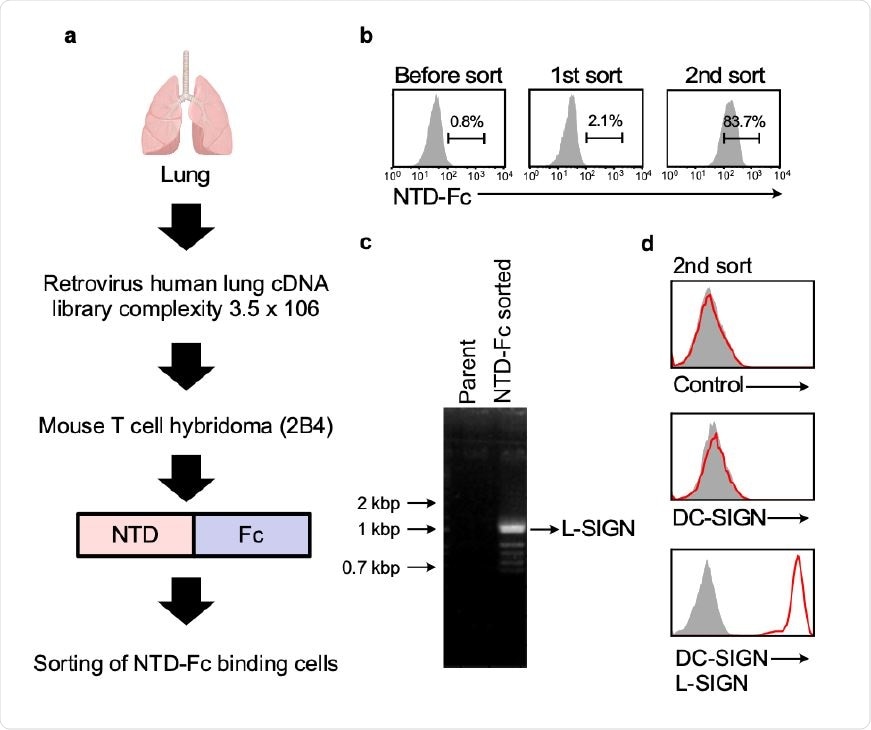
Lung cDNA library screening using the NTD-Fc of SCoV2 spike. a, The workflow of lung cDNA library screening. b, Cells transfected with retrovirus cDNA library were stained with SCoV2-NTD-Fc. Proportions of cells stained with SCoV2-NTD-Fc are shown. c, DNA agarose gel electrophoresis of cDNA library derived genes amplified from a single-cell clone stained with SCoV2-NTD-Fc. The indicated band was identified as L-SIGN. d, Single-cell clone stained with SCoV2-NTD-Fc was analyzed by anti-DC-SIGN mAb and anti-DC/L-SIGN mAb (red line) or control (shaded gray).
L-SIGN acts as NTD receptor
The researchers found that in all cases, the cells containing the viral spike protein NTD also contained the L-SIGN receptor and were stained by anti-L SIGN antibodies. This indicates, they say, “that L-SIGN is a major molecule 86 that interacts with SCoV2-NTD in the lung.” Notably, this receptor is expressed highly in the lung, despite its major site of expression being the liver.
On the other hand, no cell contained the RBD.
NTD binds to L-SIGN/DC-SIGN via unique glycans
L-SIGN and its closely related receptor DC-SIGN are both found in the lung. Another receptor called CD147 is suspected of mediating the SARS-CoV-2 infection. However, the researcher found that only NTD bound to both L-SIGN and DC-SIGN on the cell surface, but not to the ACE2 or CD147.
The RBD was found to bind to ACE2 alone. Again, both L-SIGN and DC-SIGN are ligands of intercellular adhesion molecule 3 (ICAM-3), via a sugar chain. The sugar called mannan is a ligand for both these receptors, and its presence markedly suppresses NTD binding to either of the above receptors. The researchers comment, “Unique glycans on NTD of SARS-CoV-2 on N149 are involved in the interaction with L-SIGN and DC-SIGN.”
The unique interaction between the spike NTD of SARS-CoV-2 and these receptors is mirrored by the failure of SARS-CoV NTD, or of the other human coronaviruses OC43 and HKU1, to do the same.
SIGN receptors mediate membrane fusion
Membrane fusion is essential to successful infection by enveloped viruses. The researchers found that cell-cell fusion occurred when cells infected with the viral spike protein were cocultured with cells expressing the SIGNs on their surface. This indicates that these receptors do cause membrane fusion once the cell expressing them is targeted by the virus.
Again, the researchers found that DC-SIGN, which was expressed on monocyte-derived DCs (moDCs), but SARS-CoV-2 cannot produce membrane fusion in the presence of the CD74 receptor. Thus, the SIGNs expressed in CD74-negative cells are probably responsible for mediating this infection.
Secondly, since the viral NTD binds to moDCs, it is possible that the virus attached to DC-SIGN on moDCs could infect other cells, as shown in a coculture experiment. And indeed, not only is L-SIGN expressed in lung and endothelial cells but on DCs and specialized macrophages that bear DC-SIGN, and which are also found in the lung.
The researchers comment, “Their localization in the lung further strengthens the increasingly likely role of L-SIGN and DC-SIGN as receptors of SCoV2 and may therefore be important in the pathogenesis of pneumonia.”
This infection was blocked in vitro by anti-DC-SIGN antibodies or by the glycan mannan. The ready dissemination of the virus may be explained by this phenomenon since DCs are circulating cells.
Anti-NTD/RBD antibodies neutralize SIGN-mediated SARS-CoV-2 infection
Both anti-RBD and anti-NTD antibodies blocked infection by the SARS-CoV-2 pseudovirus on SIGN-bearing cells, showing the ability of serum antibodies to neutralize the virus infection via these receptors, and indicating that both receptors play a role in successful infection.
Implications and future directions
The study thus throws some light on the possibility that SARS-CoV-2 may act through other host receptors, notably the SIGNs, to achieve infection of non-ACE2-bearing cells, and dissemination to non-pulmonary organs.
Secondly, the interaction between the NTD of this virus and the SIGNs appears unique to the current virus since neither the earlier SARS (severe acute respiratory syndrome) virus nor the seasonal coronaviruses show efficient spike binding to these receptors. In fact, the spike protein of SARS-CoV-2 has different glycan groups attached to it compared to the others, even the closely related SARS. Moreover, the N-linked glycan at residue 149, which is mostly responsible for the NTD-DC-SIGN interaction, is absent in the latter and contains a pentasaccharide motif (GlcNAc2Man3) recognized by the SIGNs.
Thirdly, the spread of the virus throughout the body, and the occurrence of severe complications, including thrombosis, may be explained by the presence of these receptors on endothelial cells as well as many other body organs. The investigators explain, “Due to their migration in the body, DCs may function as transporters [of SARS-CoV-2] by associating with the virus via DC-SIGN to infect cells expressing ACE2 or L-SIGN, which in turn facilitates virus dissemination in the host.”
Again, the presence of neutralizing antibodies found in COVID-19 patients that target SARS-CoV-2 infection by DC-SIGN receptors, indicates that the NTD is a therapeutic target. It is important to observe that most of the monoclonal antibodies from such patients fail to bind to the RBD and that the neutralizing antibodies do not prevent spike-ACE2 binding. However, the anti-RBD antibody 4A8 prevented infection of SIGN-bearing cells by the virus. Thus, the study supports the relevance of using convalescent plasma to treat these patients, especially antibodies targeting the NTD.
The study concludes, “Further analysis of L-SIGN or DC-SIGN-mediated infection would be important to understand the etiology of SARS-CoV-2-related diseases. Also, targeting SIGN-mediated infection as well as ACE2-mediated infection would be important for effective vaccine development.”
Source

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.