Metabolic disorders, characterized by the partially suppressed ability of the body's processing and distribution of macronutrients, including proteins, carbohydrates, and fats, have depicted an alarming trend of globally surging prevalence in the past few decades. Increasing global pollution levels, urbanization, poor dietary choices, and sedentary lifestyles have been identified as the main contributors to this trend, with predictions estimating metabolic disorder-associated morbidity and mortality further increasing in forthcoming decades.
Obesity and overweight are two of the most prevalent and debilitating metabolic disorders in the world today. The World Health Organization (WHO) has classified these conditions as body mass indexes (BMIs) exceeding 30 and 25, respectively, with over 1.9 billion adults suffering from the condition. The global incidence is estimated to have increased by 50% for obesity and 80% for overweight, with prediction models forecasting even worse future outcomes.
Conditions associated with abnormal weight gain are most often due to shifts in lifestyle and health behaviors, notably marked fluctuations in physical activity levels, Western-style diets, and sedentary lifestyles. Recent research suggests that additionally, genetics may play a significant role in the manifestation of overweight and obesity, as do hormonal dynamics, pharmaceutical interactions, environmental pollutants, and endocrine disruptors.
"…monogenic obesity describes a disorder promoted by single-gene mutations, usually in genes associated with endocrine regulation, that results in an obese phenotype. In parallel, epigenetic markers like DNA methylation and histone modifications exert their influence on genes intertwined with growth and metabolic processes."
The outcomes of obesity are similarly alarmingly – the condition has been associated with a host of comorbidities, including type 2 diabetes (T2D), cardiovascular disease, mental health disorders, and increased mortality risk. Recent studies have identified a link between obesity and certain cancers, with estimates of 20% of all cancer cases linked to obesity. In the United States, obesity has outcompeted smoking as the leading cause of cancers within the country. Identifying the role and mechanisms of obesity in cancer development will allow for novel therapeutic interventions targeting both conditions, resulting in significantly improved quality of life for patients and their families.
About the study
The review peruses over 280 publications investigating associations and mechanisms linking obesity and cancer. It attempts to decipher how obesity triggers cancers and allows them to persist and progress, the impacts and mechanisms of action of obesity on male prostate cancer risk, and the inherent genetic and epigenetic contributors to the transmission of obesity from fathers to their offspring.
Obesity, inflammation, and cancer
WHO defines obesity as the abnormal and excessive buildup of fat. This fat escalation profoundly impacts normal physiological processes, especially concerning the adipose tissue. Excessive fat accumulation in the adipose tissue causes adipocyte hypertrophy and hyperplasia, the former of which further promotes fat deposition, and the latter promotes a surge in adipocyte count. Together, these conditions block blood flow to adipose tissue, inducing a state of hypoxia, which, in turn, stimulates necrosis and the overexpression of pro-inflammatory factors (mainly chemokines). Over time, these factors promote localized and systemic inflammation, adipocyte rupture, and consequent death.
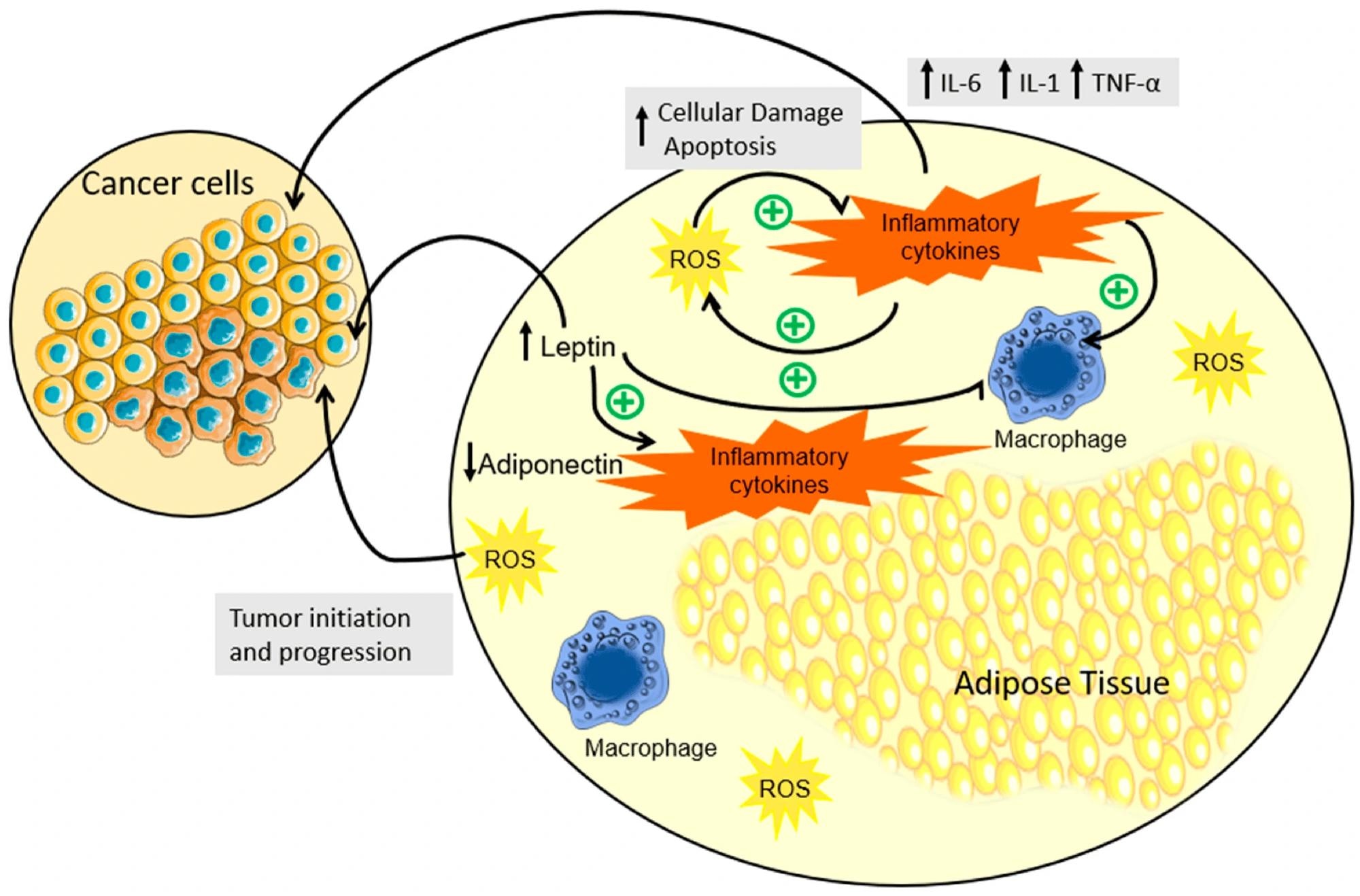 Schematic representation of inflammation, hormonal dysregulation, and OS in the adipose tissue due to obesity. In individuals with obesity, a notable expansion of adipose tissue triggers an aberrant production and secretion of cytokines, accompanied by the disruption of adipokine regulation. This cascade instigates a series of interconnected events: cytokines foster heightened ROS production, inciting apoptosis, which then exacerbates cytokine release, perpetuating a self-perpetuating cycle. This cytokine orchestration not only contributes to the perpetuation of low-grade chronic inflammation but also significantly augments the landscape for tumor development. Concurrently, elevated leptin levels in obesity correlate with heightened inflammatory cytokine levels, fostering an environment conducive to both the initiation and progression of tumors. In contrast, the diminished presence of adiponectin compounds the scenario, offering a conducive milieu for tumor development. In summary, the complex interplay between obesity, cytokine dynamics, and adipokine regulation unveils a multifaceted process that intricately contributes to chronic inflammation and the initiation and advancement of tumorigenesis. (↓) downregulation; (↑) upregulation; (+) promotion.
Schematic representation of inflammation, hormonal dysregulation, and OS in the adipose tissue due to obesity. In individuals with obesity, a notable expansion of adipose tissue triggers an aberrant production and secretion of cytokines, accompanied by the disruption of adipokine regulation. This cascade instigates a series of interconnected events: cytokines foster heightened ROS production, inciting apoptosis, which then exacerbates cytokine release, perpetuating a self-perpetuating cycle. This cytokine orchestration not only contributes to the perpetuation of low-grade chronic inflammation but also significantly augments the landscape for tumor development. Concurrently, elevated leptin levels in obesity correlate with heightened inflammatory cytokine levels, fostering an environment conducive to both the initiation and progression of tumors. In contrast, the diminished presence of adiponectin compounds the scenario, offering a conducive milieu for tumor development. In summary, the complex interplay between obesity, cytokine dynamics, and adipokine regulation unveils a multifaceted process that intricately contributes to chronic inflammation and the initiation and advancement of tumorigenesis. (↓) downregulation; (↑) upregulation; (+) promotion.
Two key chemokines involved in this cycle are interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), which have been implicated in carcinogenesis. These chemokines coax damaged adipocytes to enter a state of oxidative stress (OS) and release reactive oxygen and nitrogen species, thereby peroxidizing the surrounding lipids and triggering cell apoptosis. Apoptosis initiates a positive feedback loop wherein additional pro-inflammatory chemokines are produced, perpetuating the process.
"Concurrently, the oxidation of DNA bases promotes the occurrence of mutations and altered DNA methylation patterns, which can promote the abnormal expression of oncogenes. This underscores inflammation as a critical hallmark of cancer, which is further potentiated by obesity. Consequently, it becomes a strategic focal point for combatting the disease."
Prostate cancer
The prostate is a composite organ consisting of glandular and muscular tissue and serves as an accessory gland in the male reproductive system. Histologically, the prostate is divided into three main zones – the peripheral, central, and transition zones. Of these, the peripheral serves as the origin point for more than 70% of currently described prostate cancers and is, hence, the target for most research in the field.
The androgen receptor (AR) is the prime controller of normal prostate functioning. Notably, research has identified AR as crucial in the development and proliferation of prostate cancer (PCa). Conventional knowledge suggested that elevated levels of the male sex hormone testosterone disrupted the androgen receptor, resulting in PCa initiation. Consequently, most therapeutic interventions aimed to reduce androgen and testosterone levels and involved methods such as castration and Androgen-Deprivation Therapy (ADT). However, recent research indicates that the role of androgen hormones in PCa is more nuanced than previously thought – low testosterone levels have been implicated in the initial development of PCas, while supraphysiological levels hinder their progression.
"The therapeutic efficacy of supraphysiological testosterone levels in treating PCa can be attributed to the elevated AR expression observed in PCa cases. This upregulation of AR expression in PCa is suggested to be a compensatory mechanism aimed at counterbalancing the reduced levels of testosterone necessary for initiating tumor growth"
Obesity plays a crucial role in AR functioning and PCa development because it significantly reduces serum testosterone levels. Similar to its effects in adipose tissue, obesity promotes inflammatory responses in the prostate, thereby creating an environment conducive to the production of reactive species, which increase cellular mutation rates, thereby triggering cancers.
"…a meta-analysis study has associated obesity with a higher risk of developing aggressive phenotypes of PCa, which are resistant to traditional treatment therapies [107]. Nevertheless, excess weight does not seem to be a key risk factor for PCa, unless it is associated with altered testosterone or other androgen levels"
The role of genetics
Conventional wisdom assumed that epigenetic markings were solely inherited from the female parent due to the assumption that the protamination process protected spermatozoa from the effects of paternal epigenetic modifications. Recent research has disproved this theory and has found that between 5 and 15% of the male genome is exposed to epigenetic changes despite protamination. Furthermore, obesity- and other metabolic-focused research has found that the exposed regions are hotspots of both epigenetic modifications and contain information associated with metabolic disorders, including abnormal weight gain.
Studies in male mice have found that obesity is indeed heritable. Obese male mice were bred with healthy females, and their offspring were evaluated for physiological and genetic markers of abnormal metabolism. Female offspring were found to have increased adiposity and impaired glucose tolerance, and both female and male offspring carried a genetic predisposition to obesity despite its manifestation in only the female mice. Alarmingly, even when phenotypically healthy offspring were bred together, the F2 generation retained the epigenetic markings from the initial male obese ancestor, and in the F2 generation, both females and males expressed phenotypic obesity.
Research has previously identified the heritability of cancers. As a growing body of literature recognizes the epigenetic heritability of obesity, the prevalent fear is that these factors could summate, resulting in offspring that are overweight or obese despite observing healthy lifestyles.
Journal reference:
- Santos-Pereira, M.; Pereira, S.C.; Rebelo, I.; Spadella, M.A.; Oliveira, P.F.; Alves, M.G. Decoding the Influence of Obesity on Prostate Cancer and Its Transgenerational Impact. Nutrients 2023, 15, 4858, DOI – https://doi.org/10.3390/nu15234858, https://www.mdpi.com/2072-6643/15/23/4858