While his findings reveal the associations between hundreds of common genetic variants and hypertension, with the former’s impact on the latter varying on a per-gene basis, this review highlights the unlikelihood of ongoing and future genetic variant research discovering a novel drug or therapeutic pathway, thereby limiting the clinical relevance of the field.
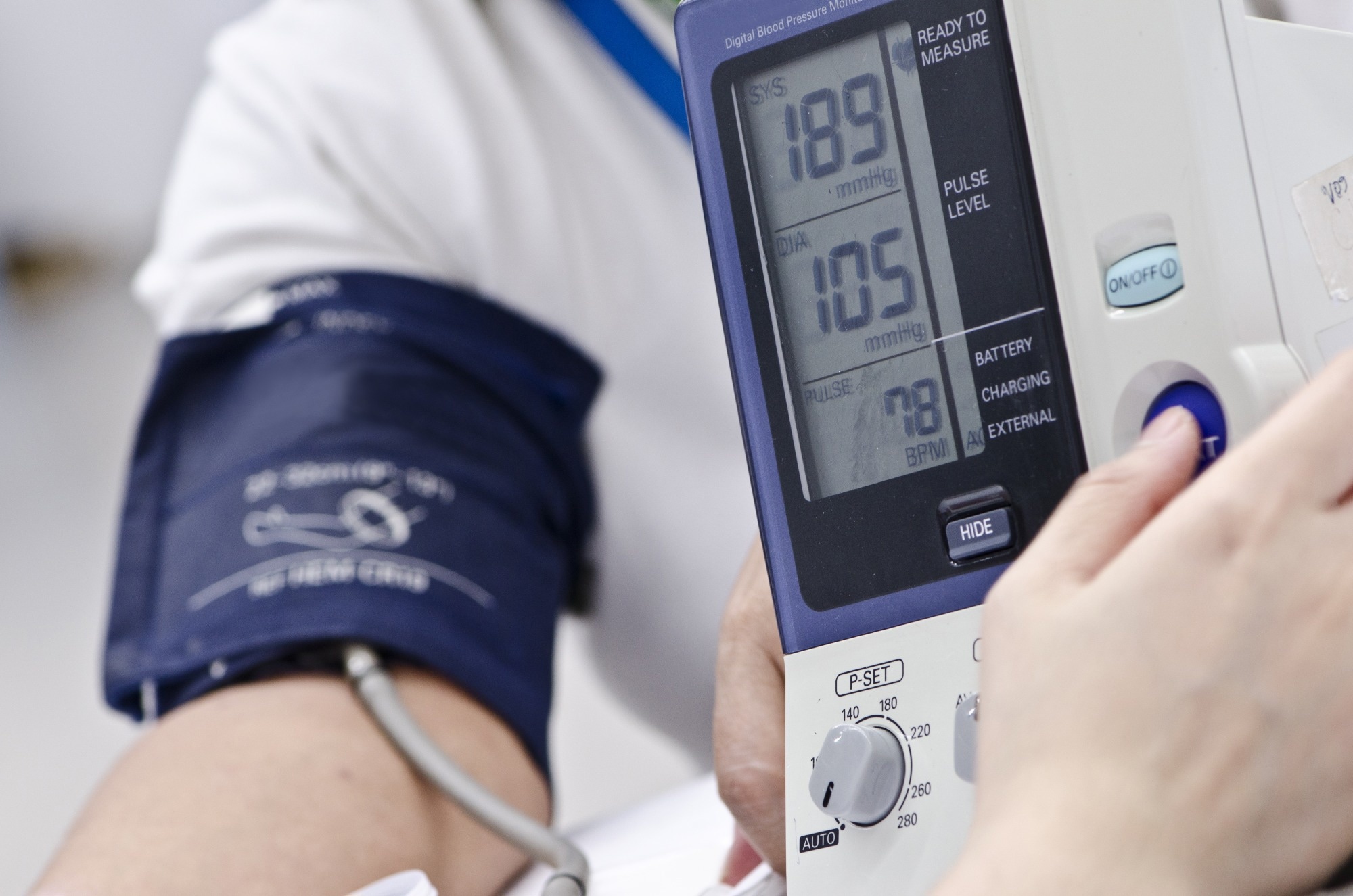 Review: Genetic Variants Associated with Hypertension Risk: Progress and Implications. Image Credit: Shine Nucha / Shutterstock
Review: Genetic Variants Associated with Hypertension Risk: Progress and Implications. Image Credit: Shine Nucha / Shutterstock
Genetic variation, the role of natural selection, and the need for this review
Despite all human being 99.9% genetically identical, mutations caused by erroneous DNA replication allow for the generation of sequence variations. In some instances, mainly when mutations occur in coding genes, these variations can profoundly impact individuals’ disease susceptibility. Research aimed at identifying these genetic variants and unraveling their clinical associations envisions a future of personalized medicine and preventive gene therapy.
“Variants with zero or minimal impacts which occurred early in human evolution can, through a process called genetic drift, grow to become common so that they may nowadays be observed in a substantial proportion of people. By contrast, any variant which causes severe disruption of a vital physiological process will tend to be quickly weeded out of the population through the process of natural selection, and hence, variants with large effects on disease risk are expected to be very rare.”
While genetic variant research has witnessed recent rapid advancements, with techniques like genome-wide association studies (GWASs) allowing for the identification of thousands of variants implicating even more genes in numerous chronic diseases, this field, unfortunately, remains in its nascency, with few reviews summarizing these advances and discussing their implications for clinicians and the general public alike. The association between genetics and hypertension is a glaring omission in this regard, given that the condition and its comorbidities represent the leading cause of human mortality worldwide.
About the study
The present study seeks to collate literature on naturally occurring genetic variants and their associations with hypertension. It touches upon the history of research within the field while focusing on the results of recent large exome-sequence projects. The review comprises more than 35 publications spanning in vitro models, in vivo clinical trials, and genome and exome-wide association studies that identify hypertension-associated variants and subsequently unravel their mechanistic underpinnings. Finally, it discusses the clinical implications of past and present genetic variant research and what this means for the layman of the future.
Research on genetic associations
Prior to current large-scale association studies, investigations regarding the associations of genetic diseases to hypertension were targeted. Counterintuitively, these investigations led to the discovery of rare genetic variants related to diseases such as congenital adrenal hyperplasia, pseudohypoaldosteronism, and familial hyperaldosteronism that may not have been discovered by current methods, the latter of which sacrifices sensitivity for broader coverage.
Their difficulties in detecting rare variants notwithstanding, genome- and exome-wide association studies are population representative and have allowed for the discovery of thousands of genetic loci associated with hypertension risk and pathology, the most notable of which are the studies conducted by the United Kingdom (UK) Biobank. The largest of these studies included more than 1 million individuals and revealed 901 associated genetic loci.
“The ability to carry out exome sequencing in large samples has now made it possible to identify every coding variant in every gene, rather than only those variants which were prespecified on the Exome Chip or those in specific genes sequenced in targeted studies.”
Thus far, more than 20 genes with single-variant hypertension associations have been discovered with substantial positive or adverse effects on disease risk and progression.
Research on functional mechanisms
Despite its specificity making research on the functional mechanisms underpinning gene-hypertension interactions substantially rarer than those aimed at establishing these genetic associations, genes, particularly those involved in blood-pressure control, have been investigated for their mechanisms of action. Studies predominantly in murine (mice) models have shown that the expression of some genetic loci can alter specific natriuretic peptide levels, altering blood pressure and, in turn, hypertension risk.
The nitric oxide (NO) signaling pathway and its associated genes have been studied from the lens of systolic blood pressure, the highlight of which is its role in blood pressure modulation, vasodilation, and pulmonary hypertension. The mechanisms underpinning the role of the dopamine beta-hydroxylase encoding DBH gene have similarly been elucidated. Some genes, such as ASXL1, FES, SMAD6, GEM, and INPPL1, are known to play a role in hypertension, but the mechanisms governing their mode of action remain elusive. Thankfully, research is ongoing to plug this knowledge gap.
Clinical implications of genetic variant hypertension research
There are three possible ways in which genetic variant research can translate into beneficial clinical outcomes – 1. Novel insights into pathogenic mechanisms, 2. Improved risk quantification, and 3. Improved treatment guidance. Unfortunately, while genetic variant research is elucidating novel mechanisms of interaction and, in rare instances, discovering hitherto unknown hypertension-associated loci, historical and current research proceeds suggest that genetic variant research is unlikely to result in novel drugs or clinical interventions.
While future clinical trials may allow for better disease prediction and inform treatment avenues, the current nascency of the field means that these trials might be years or even decades away, highlighting that the clinical implications of genetic variant research in hypertension treatment are limited, at best.