Recent advancements in medical science have significantly increased human life expectancy, leading to a gradual risNeuropathogenesis-on-chips for neurodegenerative diseasesNeuropathogenesis-on-chips for neurodegenerative diseases in the aging population globally. This is accompanied by a concomitant increase in the prevalence of age-related neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis.
Neurodegenerative diseases primarily affect the cognitive and behavioral abilities of older adults. With the accumulation of dysfunctional proteins as the primary initiating factor, these diseases share some common pathogenic characteristics, including specific neuronal loss, gliosis, neuroinflammation, oxidative stress, mitochondrial dysfunction, and early vascular damage.
Despite advancements in medical science, the development of diagnostic and therapeutic interventions for neurodegenerative diseases remains a challenging task because of the complex multifactorial pathogenesis that progresses gradually.
Microfluidic organs or organoids-on-chips have provided a unique opportunity to experimentally reproduce critical elements of distinct brain regions associated with neurodegenerative diseases. These miniaturized systems can be used for studying disease pathogenesis, drug development, drug screening, and primary biomedical research purposes.
Microfluidic chip design
The ‘Campenot chamber,’ a compartmentalized in vitro system, was the first microfluidic chip application for brain research. With two fluidically separated chambers, this device is used to study the effects of nerve growth factors on axonal growth. Later, scientists invented several miniaturized systems of neuron-glia cells, the blood-brain barrier, and the neurovascular unit.
Microfluidic chips typically contain two or more fluidically separated chambers that are connected by microchannels, porous membranes, or phase guides. These connections are required to maintain direct or indirect interactions between homogeneous or heterogeneous cell populations kept in these chambers.
The earliest microfluidic chip for the brain was designed by separating a neuronal soma from its neurites using microchannels. This design was used to study directional neurite growth. More advanced neural circuit models were developed later by incorporating multiple chambers for neuronal subpopulations.
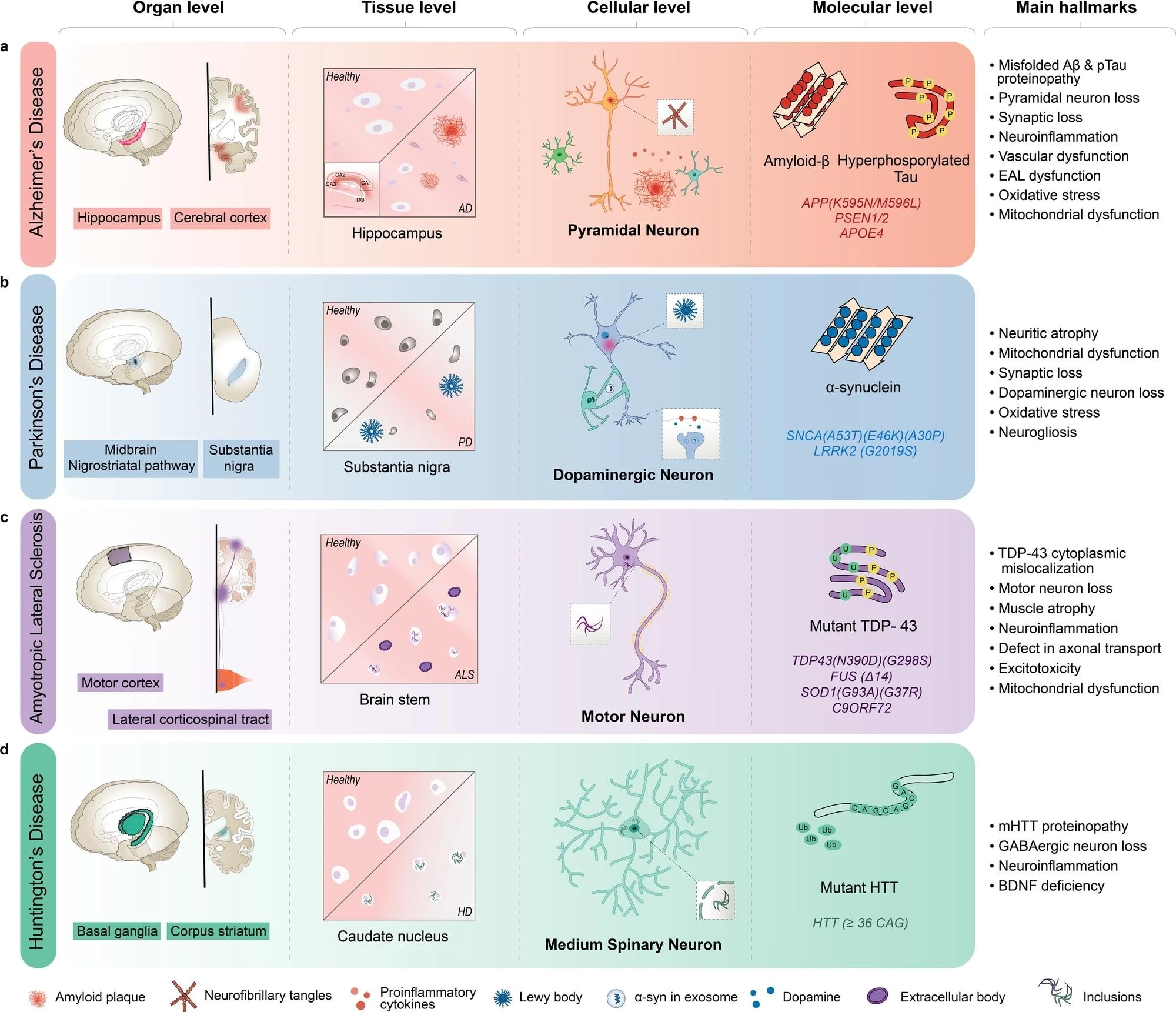 AD is characterized by the inclusion of misfolded amyloid-β (Aβ) and neurofibrillary tangles in pyramidal neurons, primarily in the hippocampus and cortex regions of the brain. b PD is characterized by Lewy body aggregates composed of misfolded α-synuclein and degeneration of dopaminergic neurons in the substantia nigra region of the brain. c ALS is characterized by including mutant TAR DNA-binding protein 43 (TDP-43) and other proteins, degeneration of motor neurons in the motor cortex and spinal cord, and muscle atrophy with dysfunctional proteins. d HD is characterized by including mutant Huntingtin protein (mHTT) and degeneration of medium spiny neurons in the basal ganglia, and corpus striatum of the brain. AD Alzheimer’s disease, ALS amyotrophic lateral sclerosis, BDNF brain-derived neurotrophic factor, EAL endosomal-autophagic-lysosomal pathway, GABA gamma-aminobutyric acid, HD Huntington’s disease, PSEN presenilin 1, SNCA synuclein alpha.
AD is characterized by the inclusion of misfolded amyloid-β (Aβ) and neurofibrillary tangles in pyramidal neurons, primarily in the hippocampus and cortex regions of the brain. b PD is characterized by Lewy body aggregates composed of misfolded α-synuclein and degeneration of dopaminergic neurons in the substantia nigra region of the brain. c ALS is characterized by including mutant TAR DNA-binding protein 43 (TDP-43) and other proteins, degeneration of motor neurons in the motor cortex and spinal cord, and muscle atrophy with dysfunctional proteins. d HD is characterized by including mutant Huntingtin protein (mHTT) and degeneration of medium spiny neurons in the basal ganglia, and corpus striatum of the brain. AD Alzheimer’s disease, ALS amyotrophic lateral sclerosis, BDNF brain-derived neurotrophic factor, EAL endosomal-autophagic-lysosomal pathway, GABA gamma-aminobutyric acid, HD Huntington’s disease, PSEN presenilin 1, SNCA synuclein alpha.
Current neuronal chips contain multiple chambers of different diameters positioned in various geometries. These models also include microchannels with patterned shapes and controlled fluid flow. These features allow for indirect and direct, asymmetric, and symmetric neuronal connections.
Extra pump systems and passive hydrostatic pressure can be incorporated into chips to control fluid flow. This helps create disease models by allowing a gradient of chemicals with varying concentrations throughout the cell compartments.
Porous membranes with different pore sizes, numbers, and positions can be used on chips as an interface between chambers to enable indirect interactions mediated by soluble chemicals and direct physical contact. This design has been used for mimicking the blood-brain barrier on chips.
Application of microfluidic chips for neurodegenerative disease pathogenesis
Microfluidic chips can be used for replicating several anatomical and physiological systems, including the neuromuscular junction, corticostriatal pathway, substantia nigra, blood-brain barrier, glymphatic system, neurovascular unit, and gut-brain axis.
To provide mechanical, structural, and biochemical cues to cells, 3D extracellular matrix gel has been introduced on chips, which allows for studying cell morphology, migration patterns, signal transduction, and gene expression in the context of neurodegenerative diseases.
Alzheimer’s disease-on-chips
The application of microfluidic chips in Alzheimer’s disease research has provided valuable insights into distinct pathogenic features, including amyloid-beta and tau protein accumulation, mitochondrial dysfunction, and neuroinflammation.
Several models of neurons-on-a-chip have been used to study tau propagation and amyloid-beta toxicity. By separating the soma and neurites, neurons-on-a-chip allow real-time visualization of proteinopathy.
A gradient chip with interstitial flow has been used to study the effect of amyloid-beta oligomers on neurons. Inflammatory cytokine-mediated migration of microglia towards Alzheimer’s disease neurons and astrocytes has been observed using a 3D static neuroinflammation-on-a-chip model.
Blood-brain barrier-on-a-chip has been developed to fully recapitulate amyloid plaque formation, neurofibrillary tangle formation, and increased permeability of the brain endothelial cells.
Dynamic neurospheroid-on-a-chip has been developed by incorporating an osmotic pump that creates a flow of exogenous amyloid-beta to study axonal degeneration and cell death.
Parkinson’s disease-on-chips
Many studies have been conducted using Parkinson’s disease-on-a-chip to primarily recapitulate alpha-synuclein-related pathogenesis. The propagation of alpha-synuclein has been studied by co-culturing neuroglioma cells that express green fluorescent protein-tagged alpha-synuclein.
A gradient chip has been developed to manipulate intracellular alpha-synuclein expression in singularly trapped yeasts in the system with a galactose gradient. Dopaminergic neurons-on-a-chip have been developed to recapitulate mitochondrial dysfunction and neural degeneration caused by Parkinson’s disease-related mutations.
Substantia nigra and vascular barrier chips have been developed by co-culturing human-induced pluripotent stem cell-derived midbrain dopaminergic neurons, primary glia cells, and brain microvascular endothelial cells in chambers separated by porous membrane. This model has been used to study blood-brain barrier-on-a-chip dysfunction, progressive neuronal loss, neuroinflammation, and astrogliosis.
Amyotrophic lateral sclerosis on-chips
Application of chemotactic and volumetric gradients on amyotrophic lateral sclerosis-on-chips has caused the successful formation of interactions between FUS-mutated motor neurons and mesangioblast-derived myotubes through microchannels.
Many pathologies of amyotrophic lateral sclerosis have been recapitulated by co-culturing TAR DNA-binding protein 43 (TDP-43)-mutated motor neuron spheroid and muscle fibers in a 3D condition between two separate chambers.
A three-chamber-chip has been developed to create metabolic interactions between superoxide dismutase-mutated astrocytes and cortical neurons through microchannels in a glutamate gradient condition.
Muscle denervation pathology of amyotrophic lateral sclerosis has been studied using an open compartmentalized neuromuscular junction device that co-cultures optogenetic motor neurons and superoxide dismutase-mutated astrocytes as a spheroid.
Huntington’s disease on-chips
Early pathologies of Parkinson’s disease have been studied by forming synaptic connections between cortical axons and striatal dendrites through microchannels of different lengths and a separate synaptic channel.
Corticostriatal on-a-chip has been developed to study how mutant huntingtin protein reduces the cortical axonal transport of brain-derived neurotrophic factors to trigger striatal neuron degeneration.