Introduction
Composition-gradient multi-angle light scattering (CG-MALS) is a biophysical technique that can be used to measure the self-association of macromolecules in solution. This technique allows fast, reproducible, and label-free measurement of this self-association. CG-MALS determines the change in weight-average molar mass as a function of concentration to ascertain the absolute affinity and stoichiometry of these interactions. This article illustrates the use of CG-MALS in measuring the isodesmic self-assembly of insulin in the absence of zinc.
Sample Preparation and Instrumentation
Samples of human insulin were prepared in a buffer up of 20 mM sodium phosphate pH 7.2, 0.1 M NaCl, 1 mM EDTA and quantified using an extinction coefficient of 1.05 AU/(g/L*cm) at 276 nm. Buffers and insulin solutions were filtered with the help of Anotop syringe-tip filters with 0.02 µm pore size by centrifugation at 2500g for a period of 15 minutes. Experiments were performed in duplicate at 25°C using two stock concentrations of 0.3 mg/mL or 3.2 mg/mL.
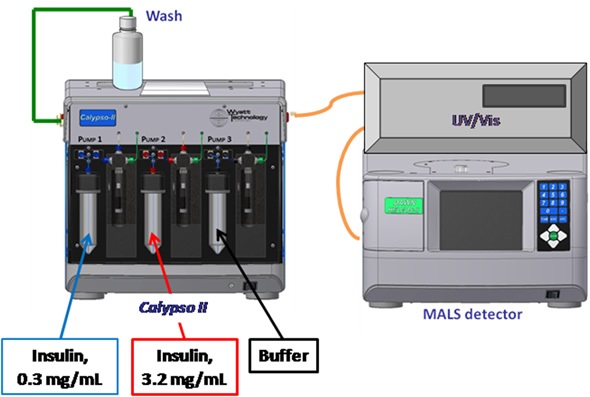
Figure 1. Calypso Hardware Set-up.
CG-MALS experiments were carried out using the Wyatt Calypso® II hardware to create concentration gradients corresponding to a miniDAWN™ TREOS® three-angle light scattering photometer (Figure 1) and a Shimadzu SPD-6AV UV/Vis spectrometer. The CALYPSO™ software was used to match the light scattering and concentration data to various self-association models to find the association scheme that best defined the data.
Results and Discussions
As anticipated, the light scattering and concentration data obtained for insulin are typical of a self-associating molecule as shown in Figure 2. While the maximum Mw (38 kDa) measured under these conditions was that of a hexamer, the rise in molar mass cannot be illustrated by a simple model of monomer-hexamer equilibrium 6I⇄I6 with an equilibrium association constant, KA = [I6]/[I]6. As shown in Figure 2, this kind of assembly overestimates the Mw by as much as 25% for concentrations between 0.2 and 2 mg/mL (34-340 µM) and underestimates the Mw by 30% for concentrations below ~0.2 mg/mL (34 µM).
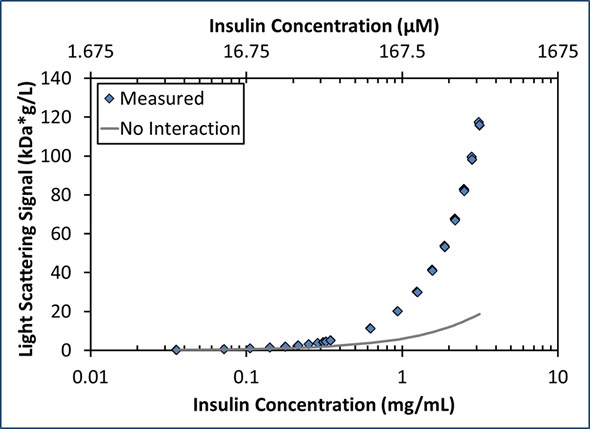
Figure 2. The measured LS signal as a function of concentration (blue diamonds) is significantly greater than the expected LS signal for a non-interacting monomer with Mw = 6 kDa (gray line).
Instead, the light scattering and concentration measurements are best matched by an isodesmic self-assembly model. According to this model, each insulin monomer combines with a growing insulin cluster with constant affinity as follows:
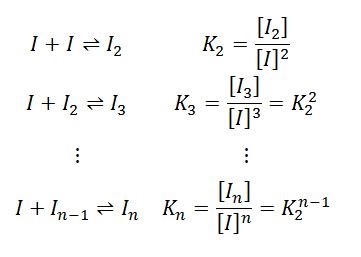
The equilibrium association constant K2 is linked to the affinity per binding site, KD=1/ K2. For the data depicted in Figures 2 and 3, isodesmic self-assembly is the best fit with affinity KD = 52 µM.
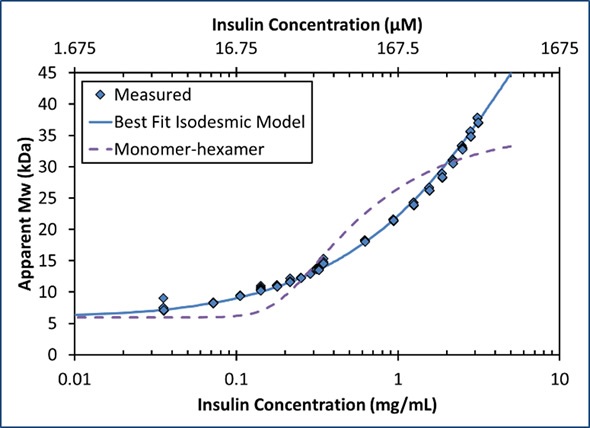
Figure 3. The increase in Mw as a function of concentration corresponds to an isodesmic self-association (solid line) and is not characteristic of a simple model of monomer-hexamer equilibrium (dashed line).
Using the self-association model, the equilibrium distribution of oligomers can be determined (Figure 4). As the total concentration reaches the isodesmic binding site affinity, the self-association of insulin molecules occurs and the monomer fraction in the solution quickly decreases. At concentrations over ~0.6 mg/mL (~100 µM), even the dimer fraction reduces as oligomers of a higher order start to form.
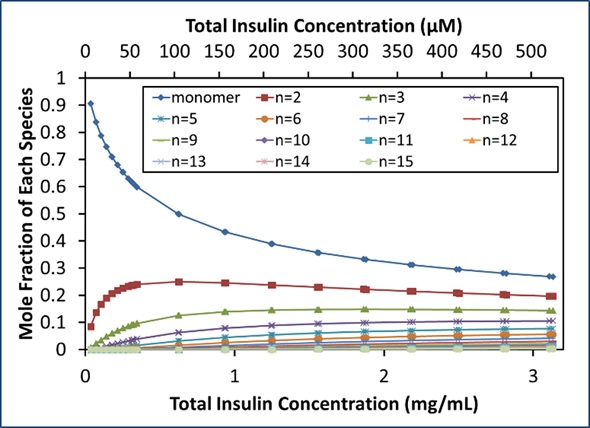
Figure 4. Equilibrium distribution of species.
At the highest concentration measured (~500 µM), monomers and dimers represent only 27% mol/mol and 20% mol/mol, respectively. Figure 4 shows the molar compositions up to 15-mers, which denote 0.3% mol/mol of the stock solution.
Conclusion
The above results clearly show that CG-MALS is capable of measuring the isodesmic self-assembly of human insulin at neutral pH in the absence of zinc. Although the overall Mw only increased by 6 times across the concentrations analyzed (~0.3-3 mg/mL, ~5-500 µM), the Mw change as a function of concentration is not defined by simple monomer-hexamer equilibrium. Instead, a more accurate picture of the complexes present at equilibrium includes higher order insulin oligomerization, with species that contain >10-mer under these conditions.
Reference
Adapted with permission from “Self-Association of Insulin Quantified by CG-MALS”, kindly submitted to Wyatt Technology Corp. by V. Pandyarajan et al., Department of Biochemistry, Case Western Reserve University. Graphs and illustrations reprinted with permission from Wyatt Technology.
- Arun K. Attri, Cristina Fernández, Allen P. Minton (2010). pH-dependent self-association of zinc-free insulin characterized by concentration-gradient static light scattering. Biophysical Chemistry, 148, 28-33.
- Arun K. Attri, Cristina Fernández, Allen P. Minton (2010). Self-association of Zn-insulin at neutral pH: Investigation by concentration gradient-static and dynamic light scattering. Biophysical Journal, 148, 23-27.
About Wyatt
 With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20% Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20% Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.