Introduction
Liposomes are synthetic vesicles composed of a lipid bilayer surrounding an aqueous core in which drugs can be contained. These vesicles therefore provide a valuable mechanism for drug delivery in the fields of clinical therapy and research. It is important to monitor liposome size and encapsulation during the research, manufacture, and quality control of these important vesicles.
Liposome size and encapsulation can be characterized using field-flow fractionation (FFF) together with quasi-elastic light scattering (QELS, also called dynamic light scattering [DLS]) and multi-angle light scattering (MALS). This article discusses the results of two liposome samples studied, one that was filled and another that was empty.
Instrumentation
For this analysis, the instruments used were the Eclipse™ FFF system and the DAWN® HELEOS® with integrated WyattQELS™ instrumentation. In addition, Wyatt’s ISIS FFF simulation software was used to optimize the FFF analysis. The online QELS was used to directly measure the hydrodynamic radius, Rh, while the HELEOS detector measured the root-mean square radius, Rg.
Analysis and Results
First, the WyattQELS detector is positioned at approximately 143°, thus extending the measurement of Rh to up to 300 nm. As shown in Figure 1, the elution time is plotted against both Rg and Rh. The results from duplicate runs demonstrate the excellent reproducibility of the FFF-MALS-QELS method.
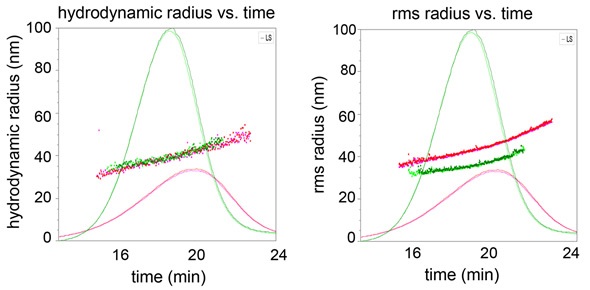
Figure 1. Hydrodynamic radius (a) and root-mean square radius (b) plotted against elution time overlaid with 90° LS signals for empty liposome sample (red) and filled liposome sample (green).
Figure 1 also shows that the Rh values are superimposed for both empty and filled liposomes, indicating that the separation is dependent on hydrodynamic radius, as expected in FFF separation. However, Rg values are not superimposed for these two liposomes, implying that they differ in their degree of encapsulation.
In order to obtain the liposomes’ internal structure from the slope of the plot, their Rh and Rg values are plotted against each other, as depicted in Figure 2. The slope of the filled liposome sample is 0.75, which is consistent with a solid sphere structure of uniform density, whilst the slope of the empty liposome sample is 1.0, consistent with a shell sphere structure.
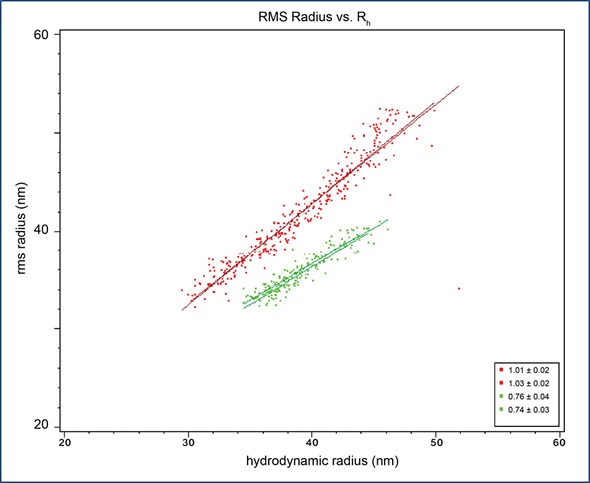
Figure 2. Root-mean square radius, Rg, plotted against hydrodynamic radius, Rh, for empty liposome sample (red) and filled liposome sample (green). The slopes for empty and filled liposomes are 1.0 and 0.75, respectively.
Conclusion
FFF-MALS-QELS is a suitable characterization technique for liposomes and other nanoparticles. This easily adaptable yet reliable method can be used to obtain data on particle count, particle size, structure, and size distribution, all without any assumption being made about particles or their composition.
Reference
Reprinted with permission from “Liposome Characterization by FFF-MALS-QELS” by Wyatt Technology Corp. Graphs and illustrations reprinted with permission from Wyatt Technology.
About Wyatt
 With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20% Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20% Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.