In liposome and lipid nanoparticle (LNP) drug-delivery applications, particle size is an important quality attribute (CQA) that can impact retention time, bioaccessibility and biodistribution. Maintaining uniform size during production is essential to consistently producing lots that pass quality control testing.
Microfluidization is a widespread method for large-scale production of liposomes within a well-defined size range. The particle size that results is dependent on numerous process parameters that can drift over the course of time, such as temperature and chamber pressure.
Present on-line and off-line control methods may hide pockets of out-of-spec material as they do not sample the whole lot. Real-time multi-angle light scattering (RT-MALS) ensures batch consistency by in-line monitoring for size of the entire batch; if the particle size no longer meets specification, the production stream can be diverted to waste.

Image Credit: Shutterstock.com/DmitryKalinovsky
Materials and Methods
Crude liposome solution containing heterogeneous liposomes with sizes from 150 nm to 800 nm was passed through a microfluidizer. At first, the internal chamber pressure was kept at 8,000 psig, then adjusted to 11,000 psig after 25 minutes to mimic a detrimental drift in the manufacturing process.
Once leaving the chamber, the solution passed through the ultraDAWN™ RT-MALS instrument at a flow rate of 4 mL/min. A lag time (RTD, residence time delay) of just 22 seconds occurred between the time product leaves the chamber and moment of measurement. OBSERVER™ software was programmed to gather ultraDAWN™ data, measure z-average radius 30 times per minute, and trigger a diversion of the exit stream if particle size deviated by 2 nm from the nominal CQA value.
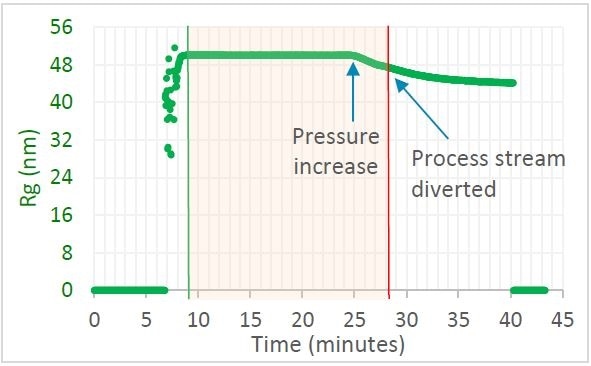
Figure 1. Trace in OBSERVER software of radius of gyration measured by ultraDAWN, indicating deviation from specification when the pressure drifted. Image Credit: Wyatt Technology
Results and Discussion
Liposome Rg was observed to be 50 nm, constant to within 0.5%, if the chamber pressure was maintained at 8,000 psig. There was a reduction in size when the internal chamber pressure was increased to 11,000 psig. OBSERVER™ successfully triggered a redirection of the process stream when the size fell below 48 nm. The entire batch would have been discarded if only the standard procedure of final QC testing was used. With RT-MALS-based monitoring and control, only around half the batch was discarded and the rest—that was collected—met specification. Additionally, highlighting the problem led to re-calibration of the chamber before the next batch was run, avoiding more loss.
Conclusions
The inclusion of in-line RT-MALS was highly valuable to this liposome production process, cutting waste of vital product and highlighting to production staff the need for immediate system maintenance.
Image Credit: Wyatt Technology
About Wyatt Technology
Wyatt Technology Corporation develops instrumentation, software and techniques for the characterization of macromolecules and nanoparticles, in solution, based on light scattering and related technologies. The physical properties determined by Wyatt’s products include absolute molar mass of proteins, polymers and other macromolecules; size and charge (zeta potential); protein-protein and other biomolecular interactions; composition of conjugated proteins and co-polymers; and macromolecular conformation.
Products and Services
Wyatt’s product line includes instruments and software for:
- on-line multi-angle light scattering (MALS), used in conjunction with size-exclusion chromatography to quantify absolute molar mass, size, conformation, conjugation and aggregation
- traditional (cuvette-based) and high-throughput (microwell plate-based) dynamic and static light scattering (DLS/SLS) to determine size and size distributions, thermal degradation and other stability-indicating parameters
- electrophoretic mobility (PALS) to determine molecular charge/zeta potential
- composition-gradient light scattering (CG-MALS) for label-free analysis of biomolecular interactions
- field-flow fractionation (AF4) for separation of macromolecules and nanoparticles from 1-1000 nm, used in conjunction with on-line MALS, DLS and other detection technologies to quantify molar mass, size, conformation and composition
Wyatt also offers, on a limited basis, sample analysis services utilizing its unique technologies.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.