Selenium is an important trace element, but it is toxic if an excess is taken. Selenosis can occur when the tolerable upper intake level of selenium exceeds 400 mg per day. Elemental selenium, as well as most metallic selenides are relatively low in toxicity due to their low bioavailabilities.
Selenites and selenates, on the other hand, are highly toxic, with a similar oxidant mode of action as that of arsenic trioxide. For humans, the chronic toxic dose of selenite is approximately 2400 to 3000 μg of selenium per day over a long period. Organic compounds such as selenocysteine, selenomethionine, methylselenocysteine, and dimethyl selenide also contain selenium. These compounds all have high bioavailabilities and are lethal in large doses (Wilber, C. G., 1980). Therefore, various environmental samples require monitoring for the presence of selenium (Tonietto, G.B., 2010).
Hyphenated techniques as modern detection systems in ion chromatography
To screen for selenite Se(IV) and selenate Se(VI) with sensitivities in the low μg/L range, a versatile approach was developed in this analysis.
Results
Ion chromatography and inductively-coupled plasma mass spectrometry (IC-ICP/MS) can be used to separate and detect Se(IV) and Se(VI). Speciation analysis is of the utmost importance, since selenium compounds strongly differ from one another in terms of toxicity. 80Se is the main isotope of Se, but m/z 80 is also the Ar-Ar-adduct and hence, the m/z 78 isotope (23.7%) of Se was measured. It was possible to take an alternative measurement on m/z 82 (8.7%), for which equal sensitivity is predicted, as shown in Figure 1.
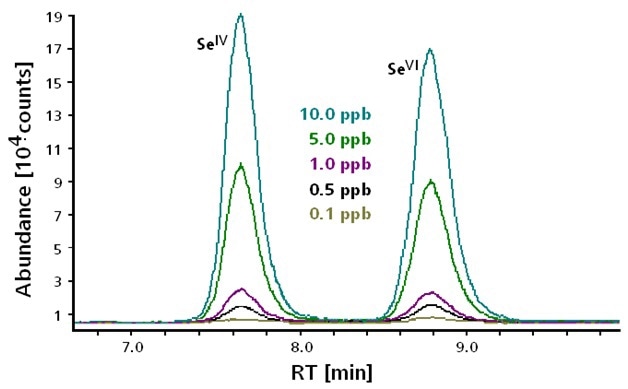
Figure 1. Separation and detection of Selenite Se(IV) and Selenate Se(VI) at concentrations between 0.1 and 10 μg/L. Column: Metrosep Anion Dual 3 - 100/4.0; eluent: 2.6 mmol/L Na2CO3, 4.0 mmol/L NaHCO3; flow rate: 0.8 mL/min; m/z 78
Simultaneous speciation of arsenic and selenium species in petroleum refinery aqueous streams
Usually, selenium exists in four oxidation states in the environment, as follows:
- Elemental selenium Se(0)
- Selenide Se(II)
- Selenite Se(IV)
- Selenate Se(VI)
Significant concentrations of selenocyanate (SeCN−) are present in some mining and oil refinery wastewaters. Since crude oil is processed in refinery operations, selenium is concentrated in the wastewater. It is difficult to treat waters that are polluted with selenium as different selenium species have very different mobilities. Conventional processes for treating wastewater such as coagulation with ferric salts cannot effectively remove SeCN−, due to its low affinity for iron hydroxide at neutral pH.
Results
In a single run, three inorganic Se species - SeCN−, Se(VI), and Se(IV) were isolated by IC using gradient elution (Figure 2) with an eluent containing 100 mmol/L NH4NO3 at pH 8.5 and adjusted by the addition of NH3.
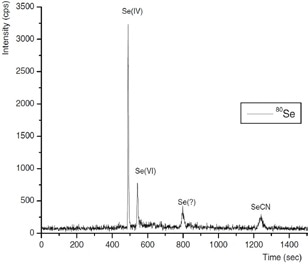
Figure 2. Ion chromatogram obtained from selenium species typically found at process wastewater inlet (condition as below)
Repeatabilities of peak position and of peak area assessments were better than 1% and ca. 3%, respectively. Detection limits were defined as 3× baseline noise and were 56, 75, and 81 ng/L for Se(VI), SeCN−, and Se(IV), respectively.
To remove selenium and arsenic interferences simultaneously, adjustments were made to key conditions of the ICP/MS octapole collision/reaction cell(C/RC), including ion lens voltages and gas flow rates. The effect on the detection limits achieved with a C/RC is greater for selenium than for arsenic.
Conclusion
The method was effective for studying the quantitative speciation of arsenic and selenium species in petroleum refinery wastewater, as shown in Figure 3.
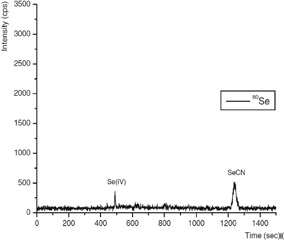
Figure 3. Ion chromatogram obtained from selenium species typically found at process wastewater inlet. Column: Metrosep A Supp 10 - 250/4.0; eluent A: 100 mmol/L NH4NO3 (pH 8.5); eluent B: ultrapure water; flow rate: 1 mL/min, gradient elution; m/z 75–83
Selenium – further applications with IC-ICP/MS
Isotope Fractionation of Selenium by Biomethylation in Microcosm Incubations of Soil - Schilling, K.; Johnson, T.M.; Wilcke, W. (2013) 352, 101–107.
References
- Aschner, M.; Syversen, T. (2005) Methylmercury: Recent advances in the understanding of its neurotoxicity. Ther. Drug Monit. 27, 278–283.
- Bianchi, A.; Calabi, L.; Corana, F.; Fontana, S.; Losi, Maiocchi, A.; Paleari, L.; Valtancoli, B. (2000) Thermodynamic and structural properties of Gd(III) complexes with polyaminopolycarboxylic ligands: Basic compounds for the development of MRI contrast agents. Coord. Chem. Rev. 204, 309–393
- Burger, J.; Gochfeld, M. (2005) Heavy metals in commercial fish in New Jersey. Environ. Res. 99(3), 403–412
- Dietary Supplement Fact Sheet: Selenium. US National Institutes of Health; Office of Dietary Supplements. Retrieved Oct 11, 2016.
- Gochfeld, M.; Burger, J. (2005) Good fish/bad fish: A composite benefit–risk by dose curve. Neurotoxicology 26(4), 511–520
- Herrmann, T. Einsatz der On-line-Kopplung von Ionenchromatographie und ICP-MS zur Bestimmung von Anionen. Diploma thesis, Philipps-Universität Marburg, Germany, 2006
- Knöll, J. Ph.D. Ultratrace determination of aminopolycarboxylic acid based chelating agents using inverse on-line coupling of IC with ICP-MS. PhD thesis, Philipps-Universität Marburg, Germany, 2013
- Knöll, J.; Seubert, A. (2012) Indirect ultra trace determination of aminopolycarboxylic acids in surface water using ion exchange chromatography coupled on-line to inductively coupled plasma mass spectrometry. Journal of Chromatography A 1270, 219-224.
- Kümmerer, K.; Ed. Pharmaceuticals in the environment: Sources, fate, effects, and risks. Springer-Verlag Berlin Heidelberg, 2008; 3rd ed.
- Künnemeyer, J.; Terborg, L.; Meermann, B.; Brauckmann, C.; Möller, I.; Scheffer, A.; Karst, U. (2009) Speciation analysis of gadolinium chelates in hospital effluents and wastewater treatment plant sewage by a novel HILIC/ICP-MS method. Environ. Sci. Technol. 43(8), 2884–2890.
- Künnemeyer, J.; Terborg, L.; Nowak, S.; Telgmann, L.; Tokmak, F.; Krämer, B. K.; Günsel, A.; Wiesmüller, G. A.; Waldeck, J.; Bremer, C.; Karst, U. (2009) Analysis of the contrast agent Magnevist and its transmetalation products in blood plasma by capillary electrophoresis / electrospray ionization time-of-flight mass spectrometry. Anal. Chem. 81(9), 3600–3607
- Levenson, C. W.; Axelrad, D. M. (2006) Too much of a good thing? Update on fish consumption and mercury exposure Nutr. Rev. 64(3), 139–145.
- Montaser, A.; Golightly, D. W.; Eds. Inductively coupled plasmas in analytical atomic spectrometry. VCH Publishers, Inc.: New York, 1992.
- Rahman, G. M. M.; Martone, N.; 'Skip' Kingston, H. M. (2012) Determination of hexavalent chromium in NIST SRM 2701 by speciated isotope dilution mass spectrometry (EPA Method 6800) using IC-ICP-MS. In Handbook of hyphenated ICP-MS applications, 2nd edition; Agilent, 2012; pp 33–35. http://www.agilent.com/cs/library/applications/5990-9473EN_icpmsSpeciationHB_lr.pdf (accessed Oct 7, 2016)
- Saranko, C. J. et al. Fact Report for toxicity of arsenite and arsenate, Florida Dept. of Health, Nov 6, 1998
- Schedlbauer, O. F.; Heumann, K. G. (2000) Biomethylation of thallium by bacteria and first determination of biogenic dimethylthallium in the ocean. Appl. Organomet. Chem.14(6), 330-340
- Wilber, C. G. (1980). Toxicology of selenium: A review.Clin. Toxicol. 17 (2), 171–230.
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.