A number of vital parameters of palm oil quality, including Free Fatty Acid content (FFA), Iodine Value (IV), moisture content, Deterioration of Bleachability Index (DOBI), and carotene content, were calculated using Vis-near-infrared (NIR) spectroscopy. This analysis demonstrates how Vis-NIR spectroscopy can precisely determine these parameters in concurrent processes.
Introduction
Palm oil is an edible vegetable oil, produced in specific geographic regions, which is extracted from the fruit of the oil palms and subsequently, distributed globally for use as an additive in biofuels, or as an ingredient in a broad spectrum of other products, including cosmetics, cleaning products, processed food, detergents and shampoos.
Palm oil’s quality is dependent on the quality of its source material and preprocessing, and distinctions are made between differing varieties. Crude Palm Oil (CPO) is derived from the fruit of the palm, while Crude Palm Kernel Oil (CPKO) is taken from the seed of the trees.
The method of production entails a preprocessing step, followed by a refining step of CPO or CPKO, during which any scent and color are eliminated. This refined product, known as Refined, Bleached and Deodorized (RBD) palm or palm kernel oil, can be divided through additional production stages into a variety of products, such as RDB olein or RBD stearin.
Each of the products stated has distinct chemical and physical properties, detailed in various norms and standards, such as Malaysian Standards MS 814 to MS 816. [1–3] Characteristic quality parameters it is necessary to determine are:
Free Fatty Acid (FFA) Content
The FFA content is a major quality factor of oils and fats. FFAs lack stability when compared to natural oil and are inclined to oxidize somewhat easily. As such, this parameter affects shelf life, storage conditions and further processing. Titration is the usual method employed to determine the FFA content.
Moisture Content
The shelf life of fats and oils is also indirectly affected by water, which leads to autocatalytic hydrolysis of oil, resulting in a higher FFA content. Additionally, bacterial activity can also result from high water content. The standard method for measuring water content is Karl Fischer titration.
Iodine Value (V)
This is correlated with the quantity of double bonds and offers insight into the degree of unsaturation of oil. Titration is the standard method for determining this parameter.
Deterioration of Bleachability Index (DOB)
This parameter determines the complexity of refining crude palm oil and is dependent on the quality of the palm oil fruits. It is frequently measured using UV/Vis spectroscopy.
Carotene Content
Carotene is the cause of the reddish color in palm oil, and originates in the stems of the palm oil fruits. The carotene content is typically determined by UV/Vis spectroscopy.
Vis-NIR spectroscopy offers an alternate method for quality control of palm oil products. As will be shown in this article, this analytical method enables each of the above listed quality parameters to be determined in under 60 seconds, using concurrent processes.
Configuration
961 samples of CPO, 345 samples of CPKO, 320 samples of RBD olein, and 320 samples of RBD stearin were employed in this analysis. The NIRS XDS Vial Heater was used at 60 °C to preheat each sample and transform it to a liquid state.
Samples were analyzed in 8 mm disposable glass in transmission mode on a Metrohm NIRS XDS RapidLiquid Analyzer over the full Vis-NIR wavelength range of 400–2500 nm. To circumvent the risk of solidification, the temperature was maintained at 60 °C.
For data acquisition and management, as well as the development of the qualification and quantification methods (Fig. 1 and Tab. 1), the software package Vision Air 2.0 Complete was employed.

Figure 1. A NIRS XDS RapidLiquid Analyzer was used to collect the spectral data of samples in transmission mode covering the full Vis-NIR wavelength range of 400–2500 nm.
Table 1. Equipment and software used
| Equipment |
Metrohm code |
| NIRS XDS RapidLiquid Analyzer |
2.921.1410 |
| NIRS 8 mm disposable glass vials |
6.7402.000 |
| Vision Air 2.0 Complete |
6.6072.208 |
| NIRS XDS Vial Heater |
2.9219.010 |
Results
The ranges of the quality parameters varied across the products tested. For example, RBD olein samples with an IV between 56 and 59.1 were outside the calibration range for IV in RBD stearin, which has IV range between 27.8 and 45.1. As such, specific models were created for each product.
These product-specific calibration models can be joined to create a single two-part operating process. The initial stage, an identity test, is a qualitative model, which detects the current product (CPKO, CPO, RBD olein or stearin). The secondary stage is where the software automatically applies the particular calibration model for the detected class.
Qualitative Analysis
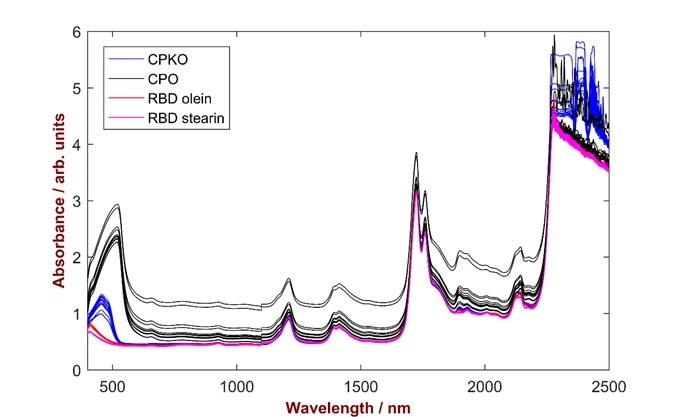
With the NIRS RapidLiquid Analyzer, spectral data can be collected from 400 nm to 2500 nm. As each product displays variations in color (Fig. 2), this extended spectral range is vital for the application.
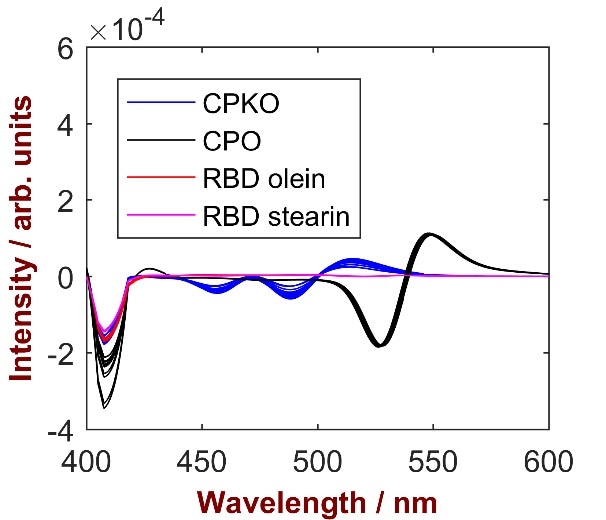
Figure 2. Second derivative spectra of different palm oil products in the spectral range between 400 and 600 nm. The observed strong difference in intensity is caused by the different color of analyzed samples.
Consequently, qualitative models were created using the entire spectral region, with second derivative pretreatment and maximum distance in wavelength space algorithm. Lastly, the library was authenticated in Vision Air Complete software.
Fig. 3 shows the results of the qualitative analysis, and makes plain that the ratio of misidentification is negligible and thus, the model is appropriate for the identification of CPO, CPKO, RBD olein or RBD stearin.
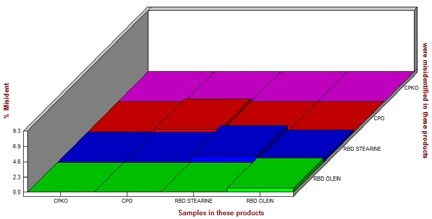
Figure 3. Results of qualitative analysis. The ratio for misidentification is close to zero.
Quantitative Analysis
The Partial Least Squares Regression (PLS) algorithm in Vision Air Complete was used to develop the quantification methods for the individual parameters and products. Depending on which pretreatment brought about the best analytical figures of merit, the data was pretreated using first or second derivatives.
Lastly, qualitative and quantitative models were joined in Vision Air Manager to form a clear and simple-to-use operating process for day-to-day analysis of varied palm oil products. The correlation plots are exemplarily displayed in Fig. 4–8. These plots show strong correlation between the values identified by the reference analytical method (x-axis) and the values projected by Vis-NIR spectroscopy (y-axis).
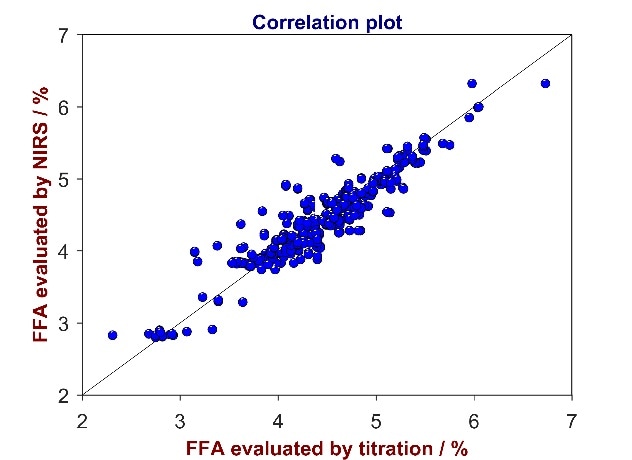
Figure 4. Correlation plot of reference values from titration versus predicted values from Vis-NIR for the analysis of FFA in CPO. The FFA content varies between 2 and 7%.
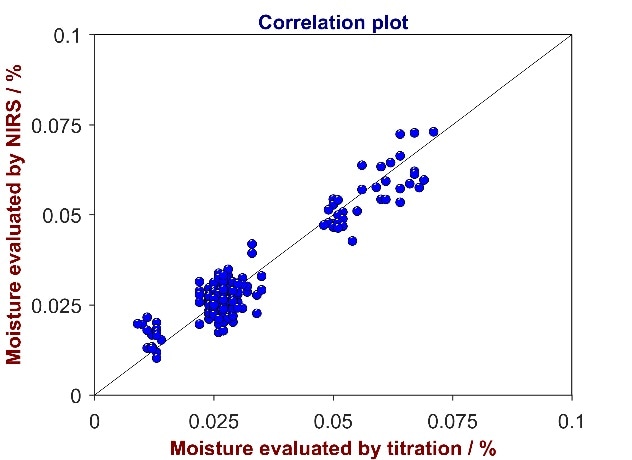
Figure 5. Correlation plot of reference values from titration versus predicted values from Vis-NIR for the analysis of moisture in RBD olein. The moisture content varies between 0 and 0.1%.
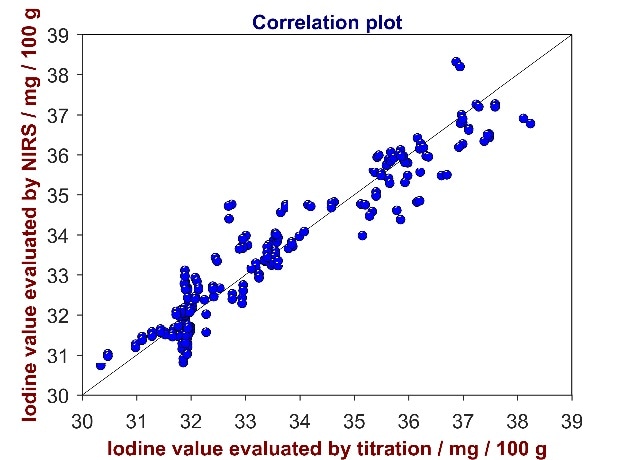
Figure 6. Correlation plot of reference values from titration versus predicted values from Vis-NIR for the analysis of iodine value in CPO. The IV varies between 30 and 39 mg/100 g sample.
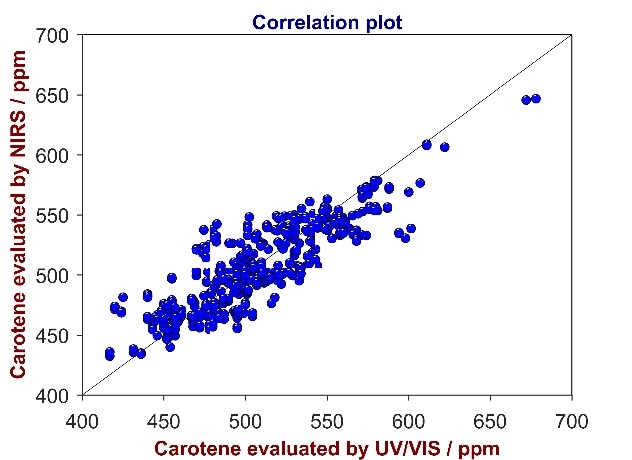
Figure 7. Correlation plot of reference values from UV/Vis spectroscopy versus predicted values from Vis-NIR for the analysis of carotene in CPO. The carotene content varies between 400 and 700 ppm.
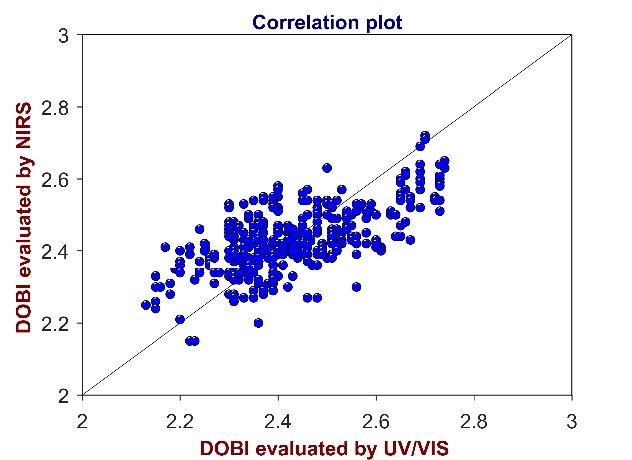
Figure 8. Correlation plot of reference values from UV/Vis spectroscopy versus predicted values from Vis-NIR for the analysis of DOBI in CPO. The DOBI varies between 2.1 and 2.8.
The analytical figures of merit for various palm oil products ratify the strong correlation results (see Tab. 2–5).
Table 2. Analytical figures of merit for CPKO analysis
| Product |
Range |
R² |
SEC |
SECV |
| FFA in % |
1–5 |
0.975 |
0.11 |
0.12 |
| IV in mg/100 g |
18–19 |
0.535 |
0.14 |
0.14 |
| Moisture in % |
0.1–0.21 |
0.495 |
0.017 |
0.018 |
Table 3. Analytical figures of merit for CPO analysis
| Product |
Range |
R² |
SEC |
SECV |
| FFA in % |
2–7 |
0.878 |
0.22 |
0.23 |
| IV in mg/100 g |
50–54 |
0.736 |
0.33 |
0.35 |
| Moisture in % |
0.06–0.56 |
0.682 |
0.045 |
0.047 |
| Carotene in ppm |
416–680 |
0.709 |
22.8 |
23.4 |
| DOBI |
2.1–2.8 |
0.435 |
0.11 |
0.12 |
Table 4. Analytical figures of merit for RBD olein analysis
| Product |
Range |
R² |
SEC |
SECV |
| FFA in % |
0–0.2 |
0.734 |
0.012 |
0.013 |
| IV in mg/100 g |
56–57.5 |
0.726 |
0.14 |
0.16 |
| Moisture in % |
0–0.1 |
0.849 |
0.005 |
0.006 |
Table 5. Analytical figures of merit for RBD stearin analysis
| Product |
Range |
R² |
SEC |
SECV |
| FFA in % |
0–0.3 |
0.931 |
0.015 |
0.016 |
| IV in mg/100 g |
30–39 |
0.909 |
0.58 |
0.60 |
| Moisture in % |
0–0.1 |
0.683 |
0.0025 |
0.0028 |
Conclusion
A Metrohm NIRS XDS RapidLiquid Analyzer was employed for quality control of a variety of palm oil products. Results demonstrating low standard errors and high accuracy were delivered through the calibration models of the Vis-NIR method.
Vis-NIR spectroscopy has been demonstrated to be a time-efficient technique for identification and quality control of CPO, CPKO, as well as RBD stearin and olein. Used alongside the intuitive Vision Air Software, this technique saves on time and costs during day-to-day analysis of palm oil products.
Additional Information
All results displayed in this article have been used in combination with the data from other comparable applications to assist in the development of precalibration for palm oil analysis.
References
- Malaysian Standard, MS 814 Palm Oil – Specification.
- Malaysian Standard, MS 815 Palm Stearin – Specification.
- Malaysian Standard, MS 816 Palm Olein – Specification.
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.