The term liquid chromatography (LC) refers to a class of physicochemical methods which are used to separate and quantify compounds in a sample. LC is made up of a liquid mobile phase, which carries the sample, and a stationary phase where the separation occurs. The stationary phase is made up of small particles in the single µm range packed in a separation column.
The separation of sample compounds depends on their relative strength of interaction with the stationary and mobile phases: compounds with a weaker relative affinity for the stationary phase will have a weaker retention on the separation column and therefore, will elute before the compounds which possess a stronger relative affinity for the stationary phase.
The compounds may be quantified by utilizing a range of different detection methods after the separation. High-performance liquid chromatography (HPLC) and on chromatography (IC) are among the liquid chromatography methods in analytical chemistry which are used most frequently.
They are vital tools for several industries, and are employed for raw material inspection, analysis of additives, and final product quality control. Even samples which are thermally sensitive can be analyzed without any issues, as both methods can be carried out at room temperature.
LC has become crucial in the production of pharmaceuticals in addition to foods and beverages and in the analysis of water – from process water and environmental waters, to sewage water. In these sectors which are strongly regulated, HPLC and IC supply selective and sensitive analyses which ensure compliance with standards and norms.
HPLC vs. IC: The Differences
The line between IC and HPLC sometimes becomes blurred in the literature. In fact, the two methods are closely related. Below, the differences between HPLC and IC are outlined.
Separation Mechanisms
In HPLC, separation is based on hydrophobic interactions (reversed phase HPLC) or hydrophilic interactions (normal-phase HPLC) of the analytes with the stationary phase.
This means the method is suitable for the separation of polar and nonpolar organic compounds. Reversed-phase HPLC is the most commonly employed type of HPLC mode with the most applications. The separation depends on organic solvents as eluents. In IC, the most crucial separation mechanism is ion exchange.
Depending on the application, other mechanisms, like ion pairing and ion exclusion are also utilized. Separation by ion exchange is reliant on chemical reactions of the ionic analytes with the ion exchanger groups of the stationary phase instead of the physical interactions as in HPLC.
Yet, the separation of non-ionic, polar compounds is equally possible buy employing IC equipment, when the right mobile phase and column are utilized. This makes IC appropriate for the separation of organic and inorganic ions and polar substances.
Eluents
HPLC separations are performed by utilizing organic solvents as eluents, typically using gradient elution to acquire good resolution for the weakly retained analytes and make the elution of strongly retained analytes faster. The samples are typically also dissolved in organic solvents to be miscible with the eluent.
The separation in ion chromatography happens in the aqueous phase, contrary to HPLC. Eluents generally consist of ultrapure water with dissolved salts or acids. Most IC separations can be performed using isocratic elution, i.e., without a gradient.
Separation Columns
Separation columns in HPLC and IC are both based on surface-modified particles as stationary phases which interact with the analytes in order to be separated. But the surface functionalization is different: the stationary phase in HPLC relies on hydrophobic (reversed-phase HPLC) or polar (normal-phase HPLC) functional groups, whereas IC is characterized by anion or cation exchanger groups.

Figure 1. IC columns differ from HPLC columns not only on the inside: IC column housings are made of chemically inert PEEK rather than stainless steel, which is susceptible to corrosion when exposed to the eluents used in IC.
Detection
The UV detector is the primary detector employed in HPLC. UV detectors can detect light-absorbing substances, as the eluate leaving the separation column is irradiated with UV light. The analyte is calculated from the difference in absorbance found between the mobile phase without sample and eluate containing analyte.
A conductivity detector is classically used in IC. By measuring the changes in the conductivity of the eluate, this detects and quantifies the analyte. Conductivity detection is a sensitive but nonselective detection method.
Table 1. Comparison of ion chromatography and high-performance liquid chromatography.
| |
HPLC |
IC |
| Analytes |
Polar and non-polar organic molecules |
Ionic and polar inorganic and organic molecules |
| Injection volume |
50–20 μL |
Variable volume up to 1000 μL |
| System |
Stainless steel, 0–50 (100*) MPa,typically 15–25 (90*) MPa |
PEEK, 0–35 MPa, typically 10–20 MPa |
| Eluent |
Organic solvents, typical gradient profile |
Ultrapure water with salt, typically isocratic method |
| Gradient |
High and low pressure gradients |
High and low pressure gradients |
| Separation |
Adsorptive effects,
→ fast with very sharp peaks |
Chemical reactions,
→ typically 15 min for 7 peaks |
| Detection |
Primarily UV |
Primarily conductivity |
| Special features |
test |
Suppression |
*Maximum pressure in Ultra-High-Performance Liquid Chromatography (UHPLC)
When HPLC Fails
Limitations of HPLC
There are some limitations to what HPLC can do, which are mainly due to the columns and the detector employed. For example, on HPLC columns, it is not possible to separate most ionic analytes. This means that HPLC is not suitable for analyses of standard cations or anions in various foods and water.
The same is true for organic acids, e.g., in beer, wine, or dairy products. In other instances, HPLC may reach its limits in the detection step: UV detection requires that the analytes absorb UV light. Analytes that are important in the analysis of food, water, and pharmaceuticals, including chloride and fluoride, do not absorb UV light, so they cannot be established using HPLC.
Overcoming the Limitations of HPLC Using IC
Ion chromatography is usually able to provide a solution when HPLC fails. IC enables separation of many analytes that cannot be separated on HPLC columns by utilizing ion exchange as separation mechanism. IC is perfect for the separation and determination of ions and polar molecules, both inorganic and organic.
In addition, analytes which do not absorb UV light, like chloride and fluoride, can be established by IC because of conductivity detection. By utilizing conductivity detection with suppression, (which will be detailed below) optimum sensitivity can be attained.
Suppression
Suppression: Increase Measuring Sensitivity
Ion chromatographic separation needs conductive buffers in the mobile phase. So, the eluate leaving the separation column is extremely conductive. Conductivity detection of the analyte ions against this high background conductivity is less than ideal.
The solution to this problem is known as suppression, which lowers the background conductivity. Ion chromatographic analyses with conductivity detection, especially anions, almost always involve suppression. Salts of weakly dissociated acids, e.g. sodium hydrogen carbonate or sodium carbonate, are utilized as eluents in ion exchange chromatography.
Throughout suppression, by means of ion exchange, the positively charged ions of the eluent are replaced with protons. The carbonic acid which is formed as a result of this is very weakly dissociated and so displays an extremely low residual conductivity. Similarly, the positively charged counterions of the analyte anions are replaced with protons.
The conductivity of the analyte-containing eluate substantially heightens as a result of this. To summarize, suppression lowers the background conductivity of the eluent, optimizes the signal-to-noise ratio, minimizes baseline noise, and heightens the sensitivity of the measuring system.
The Metrohm Suppressor Module
The patented Metrohm Suppressor Module, also known as MSM, is made up of a small rotor which contains three cartridges that are filled with cation exchanger resin (Figure 2). While the first cartridge is utilized for suppression, a regeneration step is carried out on the second cartridge.
In the meantime, the third cartridge is subject to a rinsing step. A freshly regenerated cartridge is always available for each new sample, meaning there is no idle time, due to the three-cartridge system.
The MSM has several benefits for the user compared to membrane suppressors, which are also commonly employed in IC. These benefits include compatibility with organic solvents, exceptional back pressure resistance, in addition to a lifespan of 10 or more years and a 10-year warranty to guarantee this. The regenerated suppressor unit is rinsed using the suppressed eluent after detection in the MSM.
This process is known as STREAM (Suppressor Treatment Reusing Eluent After Measurement). STREAM has many benefits: there is no need for ultrapure water for rinsing, the regenerant consumption is decreased by a factor of three, and the demand for consumables is reduced significantly, which ultimately leads to lower costs, less waste, and an eco-friendly analysis.
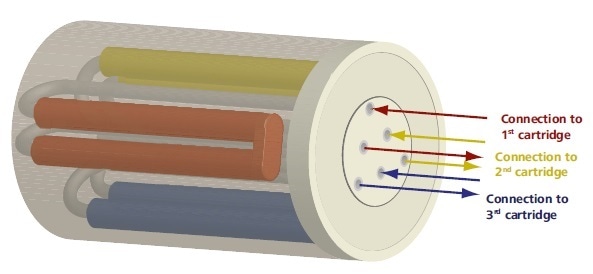
Figure 2. The Metrohm Suppressor Module contains three suppressor cartridges. A cartridge is always available for suppression while the other two undergo regeneration and rinsng, respectively.
IC Analysis of Water, Food, and Pharmaceuticals Benefits for IC Users
Ion chromatography encompasses a large scope of applications in the analysis of food, water, and pharmaceuticals. Usual samples range from strongly polluted wastewater to ultrapure water and drinking water and from environmental samples to food and beverage samples.
IC can handle the widely varying analyte concentrations of these samples, from the percent range to parts per trillion. Ion chromatography includes a large variety of sample preparation choices, many of which can be carried out inline, fully automatically. These include dialysis, ultrafiltration, and dilution.
With the Metrohm intelligent Preconcentration Technique with Matrix Elimination (MiPCT-ME), accurate determinations down to the µg/L and even ng/L ranges are permitted.
Furthermore, Metrohm IC can be paired with ‘intelligent’ sample injection methods like the Metrohm intelligent Partial Loop Technique (MiPT, Figure 3), which allows the system to measure samples across a large concentration range by adapting the injection volume of the undiluted sample.
Users of IC benefit even further from large calibration ranges, which enable them to measure samples with largely differing analyte concentrations with a single calibration. When compared to HPLC, IC has the advantage that analysis occurs in the aqueous phase, which means that organic solvents are not needed in the mobile phase.
This saves users costs both for the purchase and for the correct disposal of the solvents. Three example of applications of IC are presented in the following, one each for the sectors water, pharmaceuticals, and food and beverages.
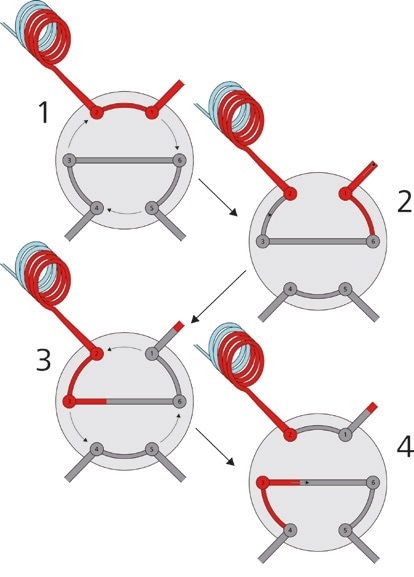
Figure 3. With the Metrohm intelligent Partial Loop Technique, it is possible to inject different sample volumes without any changes to the IC system. If a sample exceeds the calibration range, the injection volume is automatically adapted.
Application: Determination of Bromide and Chloride in Levetiracetam
Scope
Bromide and chloride occur as impurities in the drug levetiracetam, which is employed in order to treat epilepsy. While HPLC is unable to separate these anionic impurities, bromide and chloride are separated on IC columns and established with precision and high sensitivity by utilizing suppressed conductivity detection.
Sample Preparation
The sample is dissolved and diluted with ultrapure water and then subjected to five minutes of sonication. The sample is filtered through a 0.2 µm membrane before injection. Dilution and filtration can be performed automatically, inline (see below).
Instrumentation
As dilution and filtration are both needed to calculate bromide and chloride in levetiracetam, it is advised to utilize an IC system with a Dosino to handle the liquids, and an ultrafiltration cell. Using this technique, both steps can be performed inline and fully automatically. Additionally, a guard column is needed before the separation column to protect the latter from contamination.
Separation of the analytes occurs on an anion exchange column. The system is further equipped with a conductivity detector and an anion suppressor. A sample processor enables further automation of the analyses and so increases sample throughput.
Results
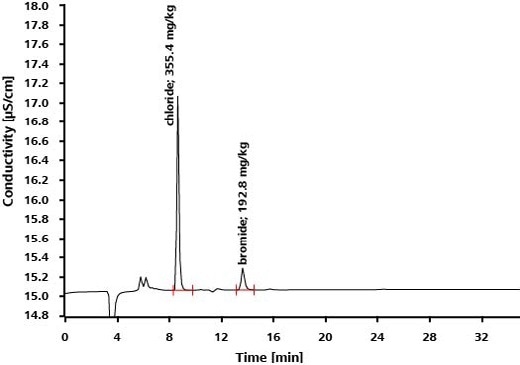
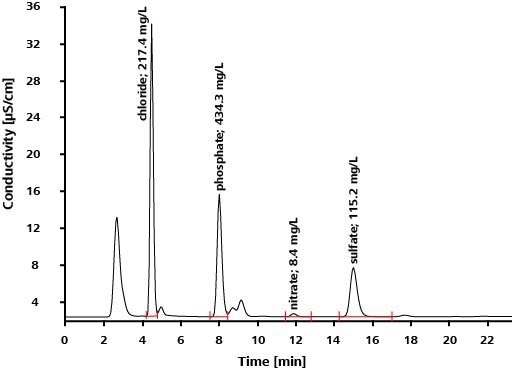
Application: Inorganic Anions in Drinking Water According to EPA 300.1 Parts A and B
Scope
EPA Method 300.1 outlines the calculation of inorganic anions in drinking water using ion chromatography. The anions covered by the norm include seven common anions (Part A) as well as three oxyhalides (Part B). The oxyhalides happen in drinking water as disinfection byproducts, see Table 2 for details. With IC, it is possible to establish both oxyhalides and anions in a single analysis run.
Table 2. Analytes described in EPA Method 300.1 Part A (common anions) and B (oxyhalides).
| Common anions |
Oxyhalides |
| Fluoride |
Chlorate |
| Chloride |
Chlorite |
| Nitrite |
Bromate |
| Nitrate |
|
| Bromide |
|
| Phosphate |
|
| Sulfate |
|
Sample Preparation
A filtration step should precede the injection to protect the column if the samples contain particles which are bigger than 0.45 microns, or if reagents contain particles which are bigger than 0.20 microns. This step can be automated (see below).
Instrumentation
If filtration is required, this can be performed inline and fully automatically by adding an ultrafiltration cell to the IC system. EPA 300.1 stipulates the use of a guard column upstream of the separation column to protect the latter. Detection is done after suppression by utilizing a conductivity cell. A sample processor enables further automation of the analyses and enhances sample throughput, in addition to reproducibility.
Results
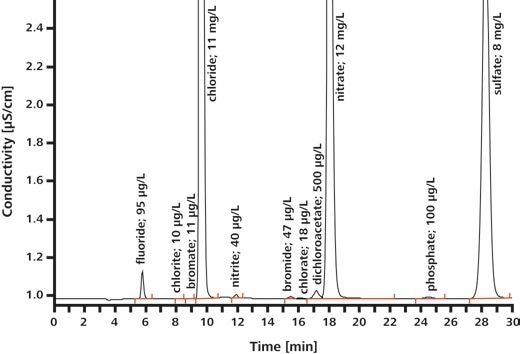
Application: Anions in Beer
Scope
In the instance of brewing beer, if they exceed the defined limits, impurities in the water utilized for brewing can end up in the finished product and influence the flavor. Consequently, anions such as nitrate, phosphate, chloride, and sulfate must be established in the quality control of beer.
Sample Preparation
Dilution of samples could be required. Additionally, beer samples must be filtered before analysis. Both steps can be done inline, automatically (see below).
Instrumentation
It is advised to use an IC system which is equipped with Metrohm Inline Dilution Technique (MIDT) and Metrohm Inline Ultrafiltration.
Using this setup, all the required sample preparation steps are carried out automatically and inline. MIDT supplies the user with the flexibility to inject samples undiluted or diluted and the possibility of automatically spiking samples. Logical dilutions are also viable: the MagIC Net software will trigger a new dilution if a sample exceeds the calibration range, followed by an injection with an adjusted dilution factor.
In addition, a guard column is needed upstream of the separation column to protect the latter from contamination. Separation of the analytes occurs on an anion exchange column. The system is further equipped with a conductivity detector and an anion suppressor. A sample processor permits further automation of the analyses and increases sample throughput.
Results
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.