Sep 16 2014
Osprey Medical Inc. (ASX: OSP) has developed a new smart syringe and LCD display system, soon to be incorporated with its existing AVERT™ System.
The enhanced System, called the AVERT™ Plus, was selected by a team of independent heart physicians from a large list of new technology applicants to be presented at an Emerging Innovative Cardiovascular Technology session at the prestigious Transcatheter Cardiovascular Therapeutics (TCT) Meeting in Washington, DC, on September 13-17. This selection enables Osprey to highlight its AVERT™ System to physicians attending one of the world’s largest gatherings of heart specialists. The meeting hosts around 12,000 attendees with over 6,000 international medical professionals in the fields of interventional cardiology and vascular medicine.
The AVERT™ Plus is a new technology that features a disposable smart syringe and reusable LCD monitor. The proprietary system offers several key benefits: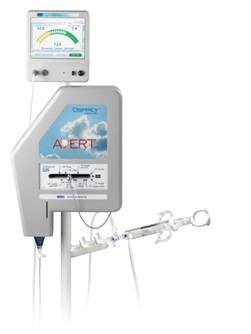
- monitors and displays the calculation of maximum dye dose levels to be used in a patient based on their kidney function before they undergo a procedure;
- automatically keeps real-time track of the amount of dye being used and the amount diverted by the AVERT™ System upon injection; and
- allows for a more accurate method of recording the dose delivered to the patient.
The AVERT™ Plus provides a simple way for doctors and hospitals to readily comply with new dosage recording guidelines that were recently issued by the US cardiology community to improve the quality of care following coronary procedures such as stenting or angioplasty.
Osprey’s President and CEO, Mike McCormick commented:
The selection of AVERT™ Plus as one of this year’s “TCT Innovation Breakthroughs” is an honour for Osprey, which we believe demonstrates the physician community’s confidence that our AVERT™ System is a significant technology.
Feedback from physicians on our AVERT™ System, both in our on-going clinical trial and our expanding commercial activities in Texas, has been very encouraging. Customers who have adopted the AVERT™ System in Texas will be upgraded to AVERT™ Plus as soon as it becomes available in the US.
AVERT™ Plus has already received European CE Mark approval and US FDA clearance is pending. The Company anticipates that the AVERT™ Plus will be available in the US in early Q1 CY 2015.