Dec 17 2014
GENFIT (Euronext: GNFT; ISIN: FR0004163111), a biopharmaceutical company at the forefront of developing therapeutic and diagnostic solutions in metabolic and inflammatory diseases, that notably affect the liver or the gastrointestinal system, today announces that a recent study provides new evidence for anti-cirrhotic effects of GFT505 in the context of NASH.
The aim of NASH treatment is to eliminate the pathology responsible for the development of fibrosis, and to prevent disease evolution to cirrhosis and its complications. In the model used for this study (Wistar rats fed a choline-deficient L-amino acid-defined (CDAA) diet + 1% cholesterol for 11 weeks), NASH is associated with the development of marked hepatic fibrosis that can progress to cirrhosis.
In this study, one group was treated with GFT505 and the results compared to those of the control group. The efficacy of GFT505 on NASH was assessed according to the same diagnostic criteria applied to the ongoing Phase 2b clinical study (GFT505-212-7: GOLDEN-505). Fibrosis was evaluated according to the scale classically used in the clinic, from Stage 0 (no fibrosis) to Stage 4 (cirrhosis).
While 91% of the control group developed NASH, only 25% of the group treated with GFT505 had NASH at the end of the treatment period.
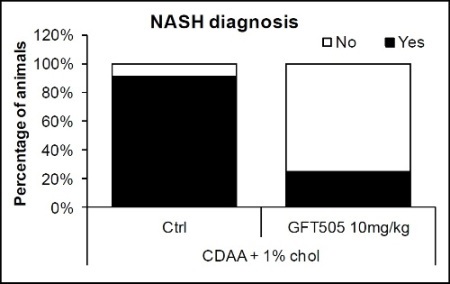
The analysis reveals that these anti-NASH effects of GFT505 are essentially due to a complete inhibition of the necrotic and inflammatory process.
Importantly, more than 90% of the control group developed advanced fibrosis (Stage 3) or cirrhosis (Stage 4), but no such cases were observed in the group treated with GFT505.
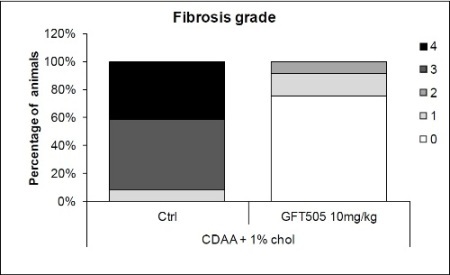
Commenting on the study, Dr. Dean W. Hum, Chief Scientific Officer of GENFIT, declared:
This data is essential since it shows that NASH treatment by GFT505 is accompanied by a blockage of the fibrotic process, resulting in protection against evolution to cirrhosis. This study alone meets all the criteria for an efficient NASH treatment in man, the ultimate aim being to protect patients from severe hepatic complications that can lead to liver transplantation or death. Complementary studies are in progress to better understand the mechanism of action of GFT505 in this model, and a scientific manuscript will be submitted for publication in 2015.