Mar 16 2016
Kanazawa University researchers find similarities in the impeded signalling between central insulin activity and glucose production in the liver for both obese mice and mice that have had the vagus nerve removed. The results are published in Cell Reports, March 2016.
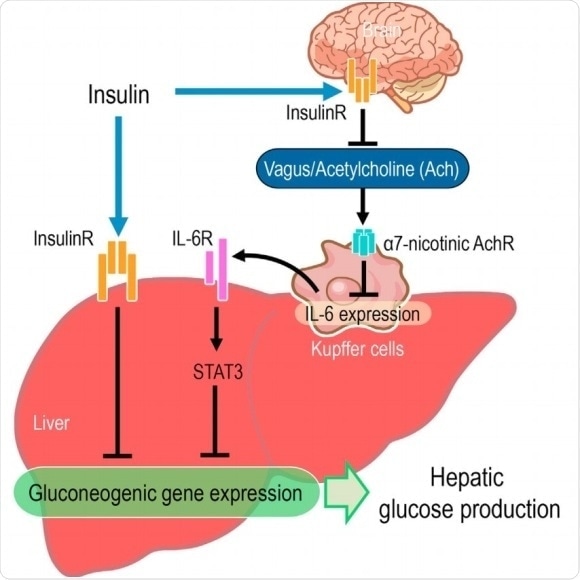
The central insulin mediated hepatic response mitigates the a7-nAchR-dependent downregulation of IL-6 expression in Kupffer cells by the vagus nerve. Kimura etal, Cell Reports 14, pp.1–13, March 15, 2016.
The vital role of insulin in controlling glucose production is often disrupted in people suffering from obesity, a condition approaching global epidemic levels. Previous work has shown that central insulin action suppresses glucose production in the liver by increasing levels of the ligand interleukin 6 (IL-6) in the liver. The ligand activates the transcription factor STAT3, which in turn suppresses gene expression of glucose-producing enzymes. However, how the liver communicates with central nervous system and the vagus nerve, which controls unconscious processes like digestion, has so far not been understood. Now a collaboration of researchers in Japan led by Hiroshi Inoue at Kanazawa University’s Institute for Frontier Science Initiative (InFiniti) has identified the molecular mechanism for this communication.
Acetylcholine is the main neurotransmitter in the vagus nerve. It also suppresses IL-6 via the α7-nicotinic acetylcholine receptor. The researchers administered insulin and monitored subsequent vagal nerve activity, as well as IL-6 levels in a type of white blood cell in the liver known as “Kupffer cells”. They noticed a decrease in vagal nerve activity accompanied by increases in IL-6 in the Kupffer cells, resulting in decreased glucose production.
The researchers then investigated the effects of administering methyllycaconitine, which prevents α7-nicotinic acetylcholine receptor activity, as well as removal of the vagal nerve. They found that while STAT3 phosphorylation and IL-6 expression in the liver increased only slightly the IL-6/STAT3 signalling response to administered insulin was lost.
The researchers compared the response in lean and obese mice and found that the administered insulin “failed to elicit changes in vagus nerve activity of high-fat diet-induced obese mice.” They conclude, “These findings suggest that the aberrant regulation of Kupffer cells via the vagus nerve and α7-nAchR-mediated cholinergic action by central insulin action may have a significant role in the pathogenesis of chronic hepatic inflammation in obesity and of dysregulation of hepatic glucose production.”