Feb 6 2018
Researchers from Kanazawa University and the National Center for Global Health and Medicine in Tokyo report, in Nature Communications, a new mechanism for regulating glucose uptake by the liver which has implications for type 2 diabetes and its treatment.
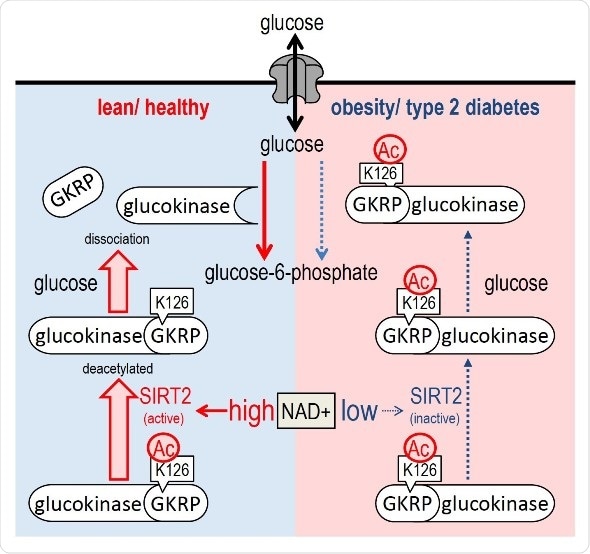
Proposed model for HGU regulation by Sirt2. NAD+/Sirt2 deacetylates K126 of GKRP, an inhibitor protein of glucokinase, thereby inducing glucose-dependent GKRP dissociation from glucokinase and enhancing HGU. However, in obesity and diabetes, impeded NAD+/Sirt2-dependent GKRP deacetylation inhibits glucokinase-GKRP dissociation and reduces HGU.
Abnormalities in glucose uptake by the liver (or hepatic glucose uptake; HGU) cause elevations in blood glucose levels following meals, a state that is known as postprandial hyperglycemia. Such abnormalities are observed in obesity and type 2 diabetes and result in an increased risk of cardiovascular complications. Although the exact mechanism of HGU impairment is unknown, there is evidence that it is mediated by abnormal regulation of the enzyme hepatic glucokinase and the glucokinase regulatory protein (GKRP).
Now, a team of Japanese researchers led by Hiroshi Inoue from Kanazawa University, Ishikawa, and collaborators from the National Centre for Global Health and Medicine, Tokyo, have identified a sirtuin enzyme (Sirt2) as a key player in regulating hepatic glucokinase through modifying GKRP, suggesting that this mechanism offers a potential therapeutic target for type 2 diabetes.
Previous reports show that the signaling molecule nicotinamide adenine dinucleotide (NAD+) governs glucose metabolism. In this paper, Inoue and colleagues used in vitro knockdown experiments to identify Sirt2 as a mediator of NAD+-dependent HGU. However, Sirt2, did not influence the gene expression levels of glucokinase and glucose-6-phosphatase, thereby implying that Sirt2 affected HGU through post-translational modifications.
In normal cells, glucokinase binds to GKRP in low glucose conditions, while the two proteins dissociate in response to elevation of glucose levels. In cells derived from diabetic mice, however, this desolation does not take place even under high glucose concentrations. In the current study, the researchers were able to reverse this perturbation by overexpressing Sirt2 and showed that Sirt2 can regulate the dissociation by directly binding to GKRP and deacetylating it (at residue K126) in a NAD+-dependent manner.
The researchers also performed experiments in mice and found that expressing a form of GKRP that could not be acetylated perturbs HGU, suggesting that acetylation of GKRP is involved in HGU and the maintenance of normal glucose levels. Furthermore, the researchers found that a decrease in NAD+-dependent Sirt2 activity and defective Sirt2-dependent deacetylation of GKRP were responsible, at least in part, for the HGU abnormality observed in obese diabetic mice.
Overall, the results indicate that NAD+ and Sirt2 regulate HGU and that Sirt2 acts through deacetylating GKRP. The authors conclude that:
These findings suggest that NAD +/Sirt2-dependent GKRP deacetylation regulation plays an important role in HGU control and that this regulation is a novel therapeutic target in type 2 diabetes and obesity and is responsible for HGU impairment."