Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), a novel RNA virus part of the betacoronavirus family. Emerging in Wuhan, China, in late 2019, SARS‐CoV‐2 spread across the globe, progressing into a full-fledged pandemic causing significant morbidity and mortality in 190 countries and territories.
SARS‐CoV‐2 infection symptoms include dry cough, fever, and gastrointestinal symptoms in most patients, while some experience a temporary loss of smell and taste. Patients with severe SARS-CoV-2 infection can experience acute respiratory distress, myocardial discomfort, stroke, and multiorgan damage during the final stages.
Currently, the FDA has approved remdesivir, an inhibitor of SARS-CoV-2 replication, to treat COVID-19, although WHO recently reported that it offers little protective effect against COVID-19.
A safe and potent drug that inhibits SARS-CoV-2 even at very low concentrations
Now, researchers from the University of California, San Francisco, and Stanford University, California, USA, reported a safe and potent antiseptic for use in humans named ethacridine, which effectively inhibits SARS-CoV-2 even at very low concentrations of EC50 ~ 0.08 µM. Their work has is published on the preprint server bioRxiv*.
Ethacridine was identified by high-throughput screening of an FDA-approved drug library in living cells with the help of a fluorescent assay. In order to identify drugs that inhibit the SARS-CoV-2 virus, the researchers redesigned green fluorescent protein (GFP) into an activity reporter of Mpro that turns fluorescent on cleavage by the active Mpro. Using this fluorescent assay, they identified many drugs that inhibit Mpro activity, and the most effective of them was ethacridine, which inhibits the production of SARS-CoV-2 through inactivation of the viral particles.
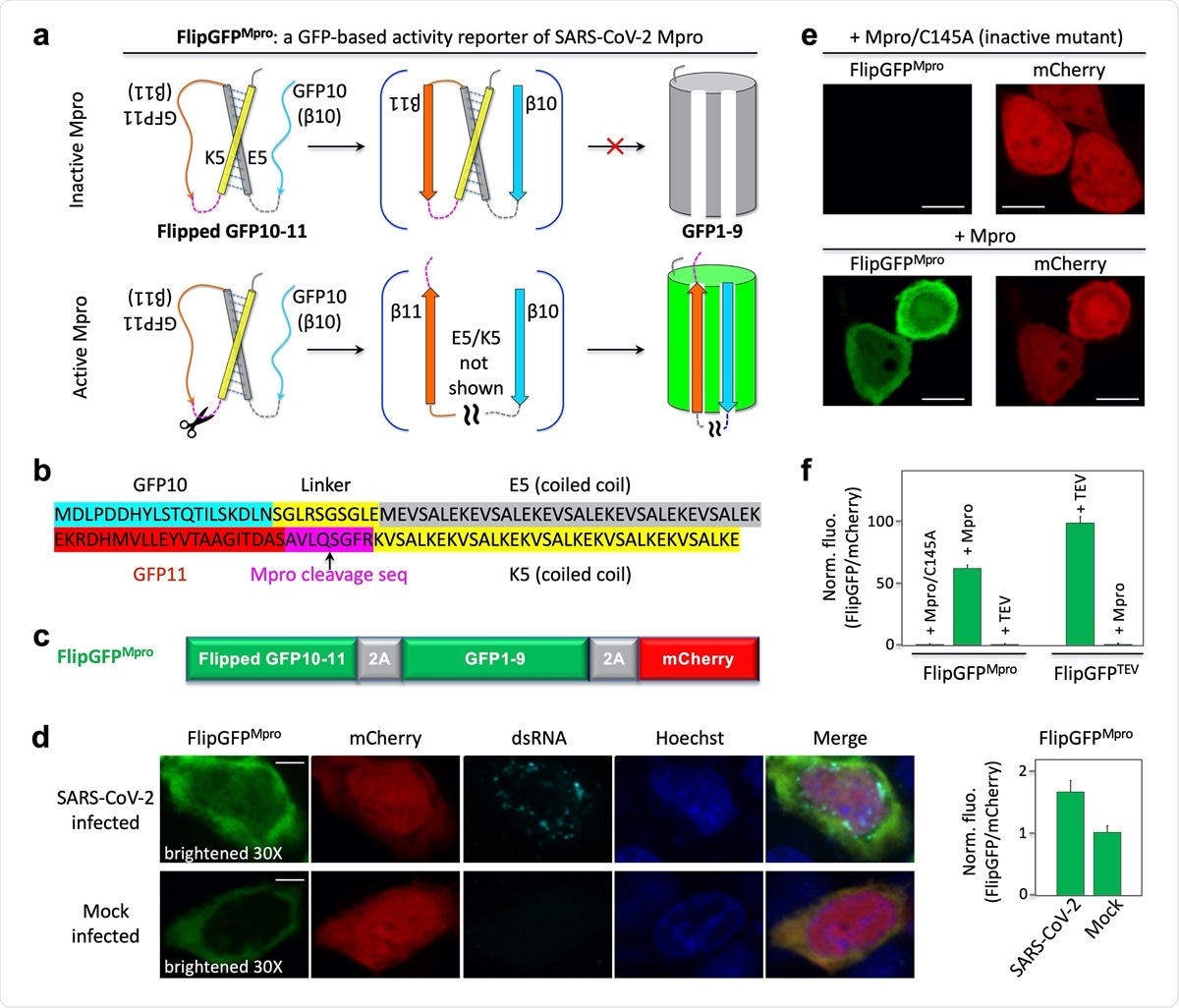
Design and demonstration of a GFP-based activity reporter of SARS-CoV2 main protease Mpro. (a) Schematic of the reporter. (b) Sequence of the flipped GFP10-11. (c) Construct of the reporter FlipGFPMpro. (d) Fluorescence images (left) and quantitative analysis (right) of SARS-CoV-2 or mock-infected HEK293T cells that co-expressed hACE2. The images in the FlipGFP channel were brightened 30-fold compared to those in (e). (e) Fluorescence images of HEK293T cells expressing FlipGFPMpro and mCherry, together with the inactive Mpro mutant C145A (upper panels) or wild type Mpro (lower panels). (f) Normalized FlipGFP fluorescence by mCherry. The ratio of FlipGFP/mCherry for the Mpro/C145A is normalized to 1. Data are mean ± SD (n = 5). FlipGFPTEV is a TEV activity reporter containing TEV cleavage sequence in FlipGFP. Scale bar: 5 μm (d); 10 μm (f).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Ethacridine blocks SARS-CoV-2 in human cell lines and is not cytotoxic in animal models
The study's findings show a novel approach against SARS-CoV-2 that inactivates viral particles, and the efficacy of this approach is expected to be independent of the cell type. Ethacridine was shown to block SARS-CoV-2 in human cell line A549ACE2 cells and in primary HNE cells. Moreover, it was not toxic in animal models, including mice, rats, and rabbits.
According to the authors, ethacridine has stronger antiviral potency compared to remdesivir and also has very little cell toxicity. Other drugs identified by this study show a similar IC50 range in inhibiting Mpro and EC50 against the SARS-CoV-2 virus. However, ethacridine showed much higher antiviral potency of EC50 ~ 0.08 μM than Mpro-inhibiting activity (IC50 ~ 3.5 μM). This shows that ethacridine's main action is not inhibiting Mpro.
"Our findings reveal a new approach against SARS-CoV-2 via inactivating viral particles, of which the efficacy is expected to be cell type-independent."
Ethacridine inactivates viral particles without affecting replication of the virus
The authors claim that their work has identified a powerful drug with a clear mode of action against the SARS-CoV-2 virus as ethacridine inactivates viral particles and thus prevents binding to the host cells. The work also provides direct evidence of SARS-CoV-2 particles losing their infectivity and ability to bind host cells after ethacridine treatment. Also, qRT-PCR data shows ethacridine does not impact viral RNA replication. Thus, the results suggest that ethacridine blocks the SARS-CoV-2 virus mainly by inactivating the viral particles without affecting the virus's replication. The further investigation focused on ultrastructural changes in viral particles and ethacridine binding on viral RNA or protein is needed to understand how ethacridine inactivates the virus.
"The detailed mechanisms of how ethacridine inactivates the viral particles will require further investigation, such as potential ultrastructural changes of viral particles and binding of ethacridine to viral RNA or protein."
Currently, ethacridine is used as a topical disinfectant, but it has already been used to treat patients with puerperal sepsis via intravenous injection. Thus, its antiviral effect can be easily validated in animal models as well as COVID-19 patients. Also, since it directly inactivates SARS-CoV-2 virus particles, ethacridine can be combined with drugs such as remdesivir, the replicase inhibitor, to treat COVID-19 patients and improve their clinical outcome.
"While currently ethacridine is mainly used as a topical wound disinfectant, it has been applied to patients for treating puerperal sepsis via intravenous injection."

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Ethacridine inhibits SARS-CoV-2 by inactivating viral particles in cellular models Xiaoquan Li, Peter Lidsky, Yinghong Xiao, Chien-Ting Wu, Miguel Garcia-Knight, Junjiao Yang, Tsuguhisa Nakayama, Jayakar V. Nayak, Peter K. Jackson, Raul Andino, Xiaokun Shu bioRxiv 2020.10.28.359042; doi: https://doi.org/10.1101/2020.10.28.359042, https://www.biorxiv.org/content/10.1101/2020.10.28.359042v1
- Peer reviewed and published scientific report.
Li, Xiaoquan, Peter V. Lidsky, Yinghong Xiao, Chien-Ting Wu, Miguel Garcia-Knight, Junjiao Yang, Tsuguhisa Nakayama, et al. 2021. “Ethacridine Inhibits SARS-CoV-2 by Inactivating Viral Particles.” Edited by Kanta Subbarao. PLOS Pathogens 17 (9): e1009898. https://doi.org/10.1371/journal.ppat.1009898. https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1009898.