After acute COVID-19, some people develop long-term symptoms known as post-acute sequelae of COVID-19 (PASC) or long COVID. Although some investigations claim that more than 30% of people infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have chronic symptoms, population-based assessments range from three to 12%. Given that SARS-CoV-2 has infected more than half of the population in the United States (US), understanding PASC is a significant public health concern.
Notably, the mechanisms behind chronic cardiopulmonary symptoms after long COVID are uncertain. Nonetheless, abnormal immune activation, endothelial dysfunction, and chronic inflammation have been linked to cardiopulmonary PASC. Characterizing phenotypes of cardiopulmonary long COVID by multimodality cardiac assessments may provide insight into possible pathways.
About the study
The present research aimed to delineate the etiology of cardiopulmonary symptoms associated with long COVID. For this, the team used multimodality evaluations, such as cardiac magnetic resonance imaging (CMR), ambulatory rhythm monitoring, echocardiography, cardiopulmonary exercise testing (CPET), and blood-based markers. The authors conducted CPET, ambulatory rhythm tracking, and CMR assessments among individuals with polymerase chain reaction (PCR)-confirmed SARS-CoV-2 infection less than one year earlier and symptomatic COVID-19 patients.
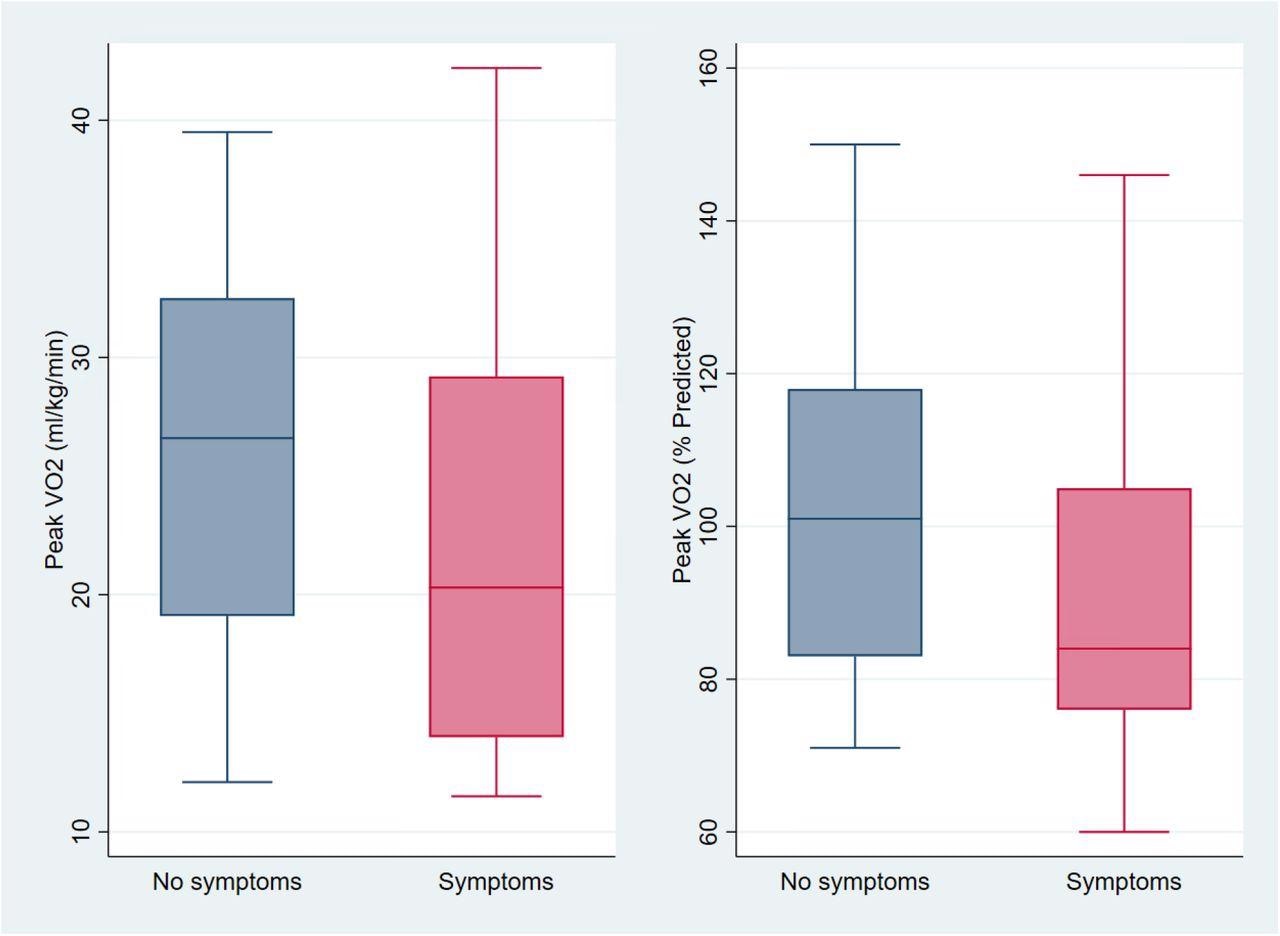
Exercise Capacity Among those with and without Cardiopulmonary Symptoms (n=39) On the left are box and whisker plots of unadjusted peak oxygen consumption (VO2 in ml/kg/min on the left and percent of predicted on the right) among those without (blue) and with chest pain, dyspnea, or palpitations (pink). Mean peak VO2 was 22.1 ml/kg/min among those with cardiopulmonary symptoms compared to 26.0 ml/kg/min among those without symptoms, a non-statistically significant difference of −3.9 ml/kg/min (95%CI −1.7 to 9.6; p=0.17) or 92% vs 103% percent predicted (difference −10.5, 95%CI −5.0 to 26.1; p=0.18). After adjustment for age, sex, hospitalization for acute COVID, BMI category, and months since SARS-CoV-2 infection, peak VO2 was 2.7 ml/kg/min lower among those reporting cardiopulmonary symptoms (95%CI −6.9 to 1.5; p=0.20) which is equivalent to 11% lower than predicted (95%CI −27 to 5, p=0.17), neither of which were statistically significant differences.
On the left are box and whisker plots of unadjusted peak oxygen consumption (VO2 in ml/kg/min on the left and percent of predicted on the right) among those without (blue) and with chest pain, dyspnea, or palpitations (pink). Mean peak VO2 was 22.1 ml/kg/min among those with cardiopulmonary symptoms compared to 26.0 ml/kg/min among those without symptoms, a non-statistically significant difference of −3.9 ml/kg/min (95%CI −1.7 to 9.6; p=0.17) or 92% vs 103% percent predicted (difference −10.5, 95%CI −5.0 to 26.1; p=0.18). After adjustment for age, sex, hospitalization for acute COVID, BMI category, and months since SARS-CoV-2 infection, peak VO2 was 2.7 ml/kg/min lower among those reporting cardiopulmonary symptoms (95%CI −6.9 to 1.5; p=0.20) which is equivalent to 11% lower than predicted (95%CI −27 to 5, p=0.17), neither of which were statistically significant differences.
In addition, the subjects were part of the long-term impact of the novel CoV (LIINC) group. The LIINC study explored SARS-COV-2 recovery in those with confirmed COVID-19 and included individuals with severe acute and asymptomatic illnesses.
Furthermore, excluded subjects in the present study included pregnant women to minimize confounding associated with expected cardiac alterations in pregnancy and those with a significant cardiopulmonary disorder, such as myocardial infarction, congenital heart disease, heart surgery, or heart failure. Additionally, subjects self-reported variables such as race, gender, income, education, and ethnicity. The team adjusted for confounders and utilized linear regression and logistic models to compare people with and without cardiopulmonary symptoms such as chest pain, dyspnea, and palpitations.
Findings
According to the study results, 46 subjects had a minimum of one advanced cardiac test at about 17 months following COVID-19 among the 120 individuals studied. In addition, the median age of the subjects was 52, 18 were female, and six were hospitalized for severe acute SARS-CoV-2 infection.
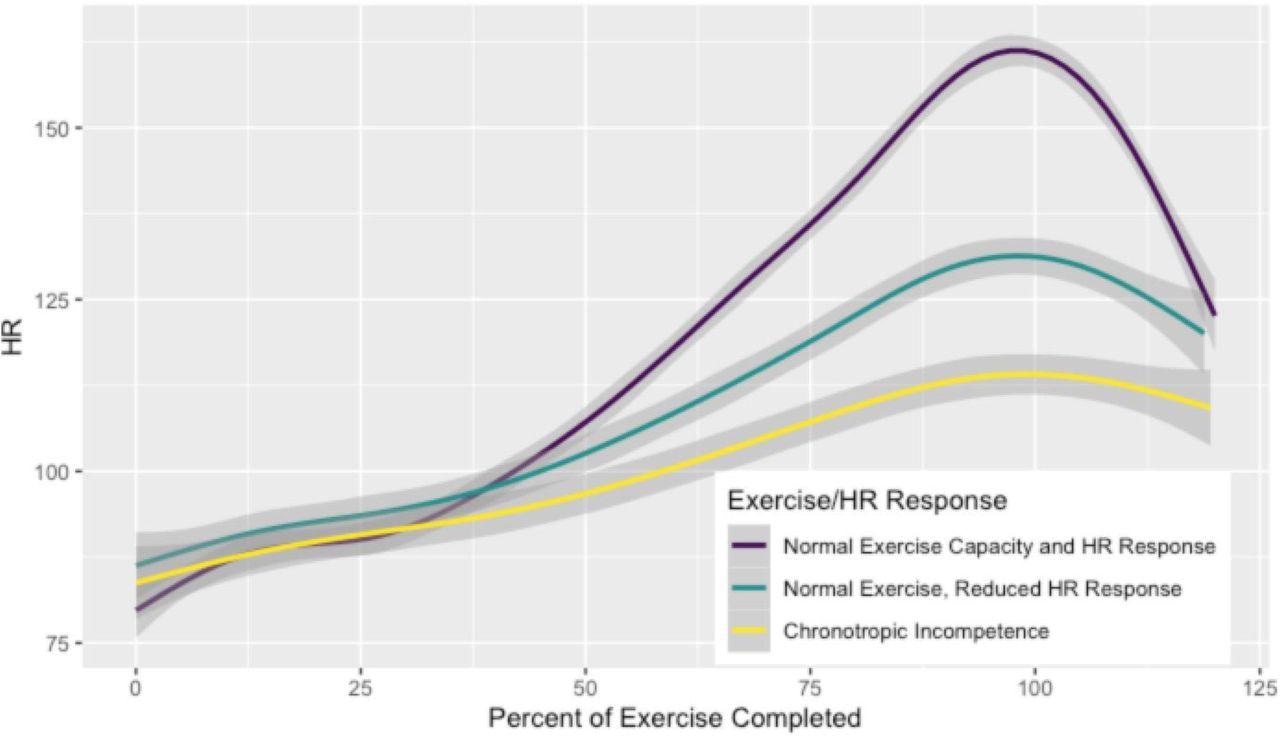
Heart Rate During Exercise by Chronotropic Response These lines represent the average heart rate at a given percentage of exercise completed classified by normal exercise capacity and chronotropic response during exercise on the top in purple (peak VO2≥85% predicted and AHRR ≥80%; R2 0.89), normal exercise capacity with reduced chronotropic response in teal (peak VO2≥85% predicted and AHRR <80%; R2 0.90), and reduced exercise capacity with chronotropic incompetence in yellow (peak VO2<85% predicted and AHRR <80%; R2 0.75). Gray bars represent 95% confidence intervals for each fitted line. Results are similar when plotting %APMHR or AHRR instead of absolute heart rate (Supplemental figure 1).
In the 39 CMR subjects, low right ventricular (RV) volume and stroke volume, and increased extracellular volume were observed in participants with cardiopulmonary symptoms. However, there was no proof of augmentation in late-gadolinium or variations in T1 or T2 mapping.
In the ambulatory tracking group, the team found no arrhythmias. On the contrary, on 39 CPET participants, 13 out of 15 with lower exercise ability reported fatigue or cardiopulmonary symptoms. The controlled peak oxygen consumption rate (VO2) was reduced by 11% of predicted values or 2.7 ml/kg/min in individuals with cardiopulmonary symptoms, such as dyspnea and chest pain. Moreover, the corrected variation in peak VO2 was -21% of anticipated values or -5.9 ml/kg/min considering fatigue and cardiopulmonary symptoms.
Further, nine of the 15 volunteers with lower peak VO2 had chronotropic incompetence as their principal aberration. A controlled heart rate reserve of less than 80% was linked with lower exercise ability. Those with chronotropic incompetence reported increased high sensitivity C reactive protein (hsCRP), reduced heart rate variability, and lower heart rate recovery, all of which are signs of autonomic dysfunction.
Conclusions
Altogether, the study findings showed that individuals with COVID-19 history and persisting cardiopulmonary symptoms 18 months after SARS-CoV-2 infection have poor exercise ability. The researchers found that reduced exercise ability was linked to chronotropic incompetence in PASC. Additionally, data from ambulatory rhythm tracking indicated higher hsCRP levels and autonomic dysfunction as a justification for cardiopulmonary PASC.
Besides, numerous subjects had trace or minor pericardial effusions. Yet, the investigators found no indication of past or continuing myocarditis. They mentioned that apart from myocarditis, these pericardial effusions were correlated to hsCRP. Moreover, the team suggested that further research into the processes of cardiopulmonary PASC should incorporate an examination of the autonomic nervous system to discover possible treatment targets.
To conclude, the current work demonstrated that after COVID-19, hsCRP and poorer exercise capacity and heart rate response to exercise were linked to cardiovascular symptoms persisting for more than a year. Besides, the findings indicated that cardiopulmonary long COVID might be caused by persistent autonomic dysfunction and inflammation.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Reduced Exercise Capacity, Chronotropic Incompetence, Inflammation and Symptoms in Post-Acute COVID-19; Matthew S Durstenfeld, Michael J Peluso, Punita Kaveti, Christopher Hill, Danny Li, Erica Sander, Shreya Swaminathan, Victor M Arechiga, Kaiwen Sun, Kaiwen Sun, Yifei Ma, Victor Zepeda, Scott Lu, Sarah A Goldberg, Rebecca Hoh, Sithu Win, J. Daniel Kelly, Timothy J Henrich, Jeffrey N Martin, Yoo Jin Lee, Mandar A Aras, Carlin S Long, Donald J Grandis, Steven G Deeks, Priscilla Y Hsue. medRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.05.17.22275235, https://www.medrxiv.org/content/10.1101/2022.05.17.22275235v1
- Peer reviewed and published scientific report.
Durstenfeld, Matthew S, Michael J Peluso, Punita Kaveti, Christopher Hill, Danny Li, Erica Sander, Shreya Swaminathan, et al. 2023. “Reduced Exercise Capacity, Chronotropic Incompetence, and Early Systemic Inflammation in Cardiopulmonary Phenotype Long COVID.” The Journal of Infectious Diseases, May. https://doi.org/10.1093/infdis/jiad131. https://academic.oup.com/jid/advance-article/doi/10.1093/infdis/jiad131/7159960.