The development of antimicrobial resistance in pathogens is rapidly becoming a public health concern of the same or possibly higher magnitude as malaria or human immunodeficiency virus (HIV). However, the process of developing vaccines is tedious and expensive, and in the case of antimicrobial-resistant pathogens, it is made worse by inadequate information on correlates of protection.
In the case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the process of vaccine development was significantly accelerated by the discovery of antibodies that could bind to the spike protein of the virus, preventing it from binding to the host angiotensin-converting enzyme-2 receptor. This discovery also indicated that neutralizing antibody titers could be used as correlates of protection since they indicated the clinical efficacy of the vaccine.
For most antimicrobial-resistant pathogens, the mechanisms through which vaccines can protect the host remain unknown. While immunomics, proteomics, and genomics are being extensively used to develop vaccines against antimicrobial-resistant pathogens, the dearth of information on correlates of protection continues to present the risk of jeopardizing late-stage clinical trials.
About the study
In the present study, the researchers presented a method of Reverse Vaccine Development, a new paradigm for vaccine development that requires information on the efficacy of the vaccine and the immune responses to be generated much earlier in the vaccine development process so that the correlates of protection can be identified early on instead of closer to phase III trials. They also implemented this paradigm to evaluate a vaccine against the antimicrobial bacteria Staphylococcus aureus.
The process is called Reverse Vaccine Development since the order of information procurement on vaccine efficacy is reversed as compared to the typical procedure of vaccine development. This information is obtained from populations that are already experiencing a high incidence of antimicrobial-resistant pathogenic infections instead of the population that will eventually get vaccinated.
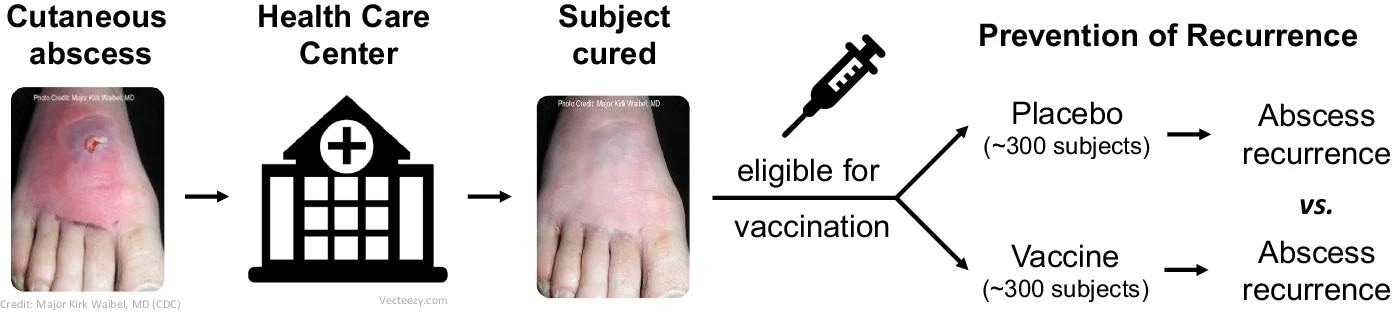
Paradigm clinical trial design (phase 1/2) in Reverse Vaccine Development: S. aureus vaccine in subjects at high risk of SSTI.
Given that animal models have not been unreliable in vaccine development against antimicrobial-resistant pathogens, using high-risk populations helps compare the immune responses of unprotected and protected individuals, which can provide data on correlates of protection.
The efforts to develop vaccines against S. aureus have yielded four candidate vaccines that target various antigens and use four different protection mechanisms. Based on the results from animal model studies and in-vitro assays, the vaccines were advanced to phase I and II clinical trials. The vaccines passed the safety assessments and elicited satisfactory antibody titers. However, the phase III efficacy trials for these vaccines failed, indicating a lack of adequate information on correlates of protection.
To circumvent such problems, the researchers in this study applied the paradigm of Reverse Vaccine Development to design a randomized, observer-blinded, placebo-controlled phase I and II trials to assess the immunogenicity, safety, and efficacy of the candidate vaccine developed against S. aureus by GSK.
Results
The study discussed how Reverse Vaccine Development differs from the traditional vaccine development process by beginning in phase I or II trials that evaluate the efficacy, immunogenicity, and safety of the vaccine instead of efficacy evaluation in phase III trials. This ensures that potential problems associated with correlates of protection are identified early in the vaccine development process and do not result in the failure of the vaccine towards the end stages when considerable resources have been invested in the process.
Phase I safety trials with and without adjuvant are often conducted if the vaccine is being developed for the first time for humans, and based on the results of the phase I safety assessments, the trial proceeds into phase II to evaluate the efficacy and immunogenicity. Comparing the immune responses elicited by the vaccine among unprotected and protected groups can help identify correlates of protection, which can then be used to formulate, schedule, and facilitate vaccine efficacy assessments in the general populations and refine the vaccine dosage.
The researchers discussed in detail the various parameters that need to be evaluated when correlates of protection are being explored. These included serology, cellular responses, immunological signals, transcriptional profiles, memory immune cell responses, and background immunity.
Conclusions
To summarize, the study described a novel vaccine development paradigm that involves conducting phase I and II trials in populations that are at high risk of contracting the target antimicrobial-resistant pathogen to understand the correlates of protection before the development process progresses to phase III trials and risks failure. This method could circumvent grave problems in vaccine development, such as exposure to inefficacious vaccines and the loss of resource investment.
Journal reference:
- Bagnoli, F., Galgani, I., Kumaran, V. V., & Phogat, S. (2024). Reverse development of vaccines against antimicrobial-resistant pathogens. Npj Vaccines, 9(1), 71. 10.1038/s41541024008584, https://www.nature.com/articles/s41541-024-00858-4