The coronavirus disease (COVID-19) pandemic is actively spreading worldwide, affecting 188 countries and territories. At the beginning of the health crisis, Italy was one of the hardest-hit countries. Now the country sits 18th on the country list for confirmed cases with a case toll of 260,298 infections and over 35,000 deaths.
Though the infection rate has slowed in the country, scientists have started human trials of a potential vaccine against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19 disease.
Rome's Lazzaro Spallanzani Institute, a hospital specializing in infectious diseases will conduct trials on 90 volunteers in the coming weeks, joining a global effort to develop an immune response to the virus.
The scientists hope the vaccine will trigger an immune response to fight the infection. Europe, one of the hardest-hit regions of the pandemic has seen resurging cases. If the vaccine is safe and effective, the scientists hope the vaccine will become available for the public by mid-2021.
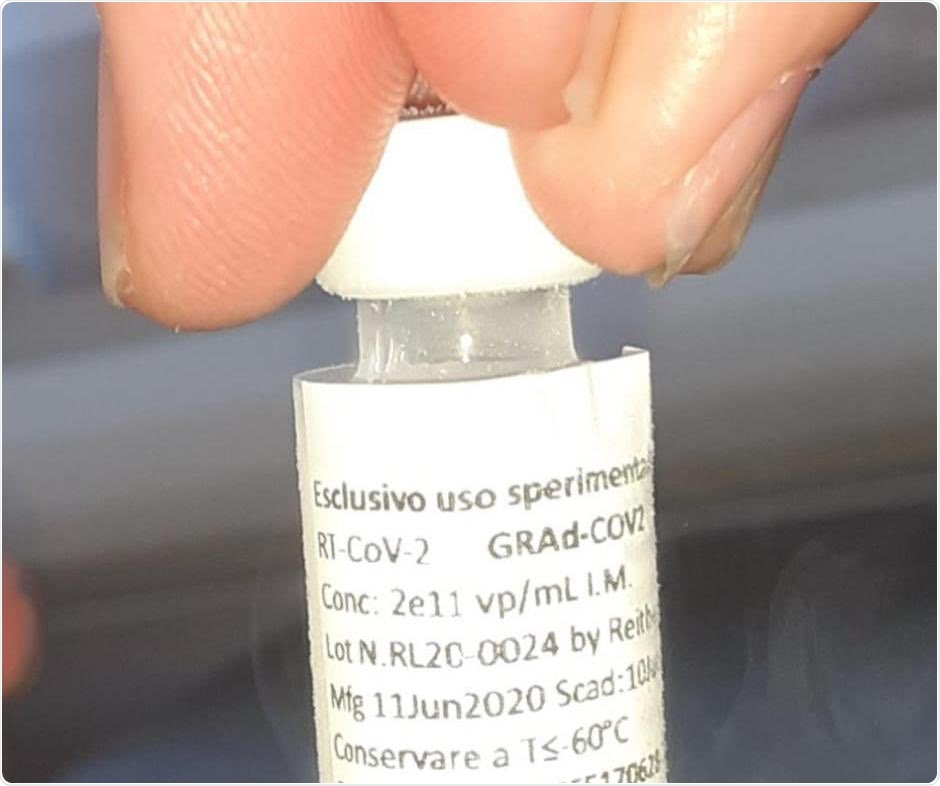
Image credit: Nicola Zingaretti
The vaccine
The vaccine, called GRAd-COV2, was developed by ReiThera, an Italy-biotech company. The vaccine encodes the full-length spike protein of the SARS-CoV-2 and is based on a novel replication-defective gorilla adenoviral (GRAd).
The vaccine technology generated a strong humoral and cellular immune response in pre-clinical studies and has been proven effective. Now, the vaccine is ready to be tested in humans, which is expected to induce strong immune responses based on low pre-existing immunity to the vector in humans.
The other vaccines based on the simian adenoviral vectors, such as the Chimpanzee adenoviral vectors (ChAd), have been observed and evaluated in Phase 1 and 2 clinical trials.
Simian adenoviral (Sad) vectors have been widely used as delivery agents for genetic vaccine candidates against a multitude of infectious diseases, such as the Respiratory Syncytial Virus (RSV) and the Ebola virus, which has ravaged through West Africa in 2014.
"Reithera's novel simian adenovirus (GRAd) belongs to species C adenovirus that is considered the most potent vaccine carriers. It has low seroprevalence in humans, compared to other simian Ads (adenoviruses) and human Ads, and an immunological potency comparable if not higher than the most potent other Ads," said in the vaccine's website.
The trial
The clinical trial, which has stared on August 24, will enroll 90 healthy volunteers in two age cohorts – the 18 to 55 years old group, and the 65 to 85 years old group. Each of the groups will be divided into three study arms of 15 volunteers, who will receive one of three increasing doses of the vaccine. All the participants will be monitored and assessed for over 24 weeks.
The vaccine's phase 1 trial is now being performed by the Lazzaro Spallanzani National Institute for Infectious Diseases under the sponsorship of ReiThera. The main goal of the trial is to see if the potential vaccine is safe and well-tolerated in humans and if the vaccine can induce immune responses, including both antibodies and T cells, against the SARS-CoV-2.
If the first leg of the trial will be promising, a more extensive international phase 2 and phase 3 will commence by the end of 2020 in countries where the pandemic is active and spreading.
"This study is the first important step in the clinical development of our novel GRAd-COV2 vaccine against COVID-19. We are proud to undertake this trial in Italy where the impact of COVID-19 has been felt particularly hard," Stefano Colloca, ReiThera's Chief Technology Officer, said
"The cutting-edge science behind our approach is backed by many years of pioneering research on adenoviral vector technologies with pre-clinical and clinical data generated with a single-dose vaccine in other serious infectious diseases demonstrating potent humoral and cellular immune responses. This feature makes our technology platform suitable for an outbreak situation such as COVID-19 and has clear advantages in terms of manufacturing and compliance," he added.
The vaccine race
According to the World Health Organization (WHO), 139 vaccines are currently in the pre-clinical stage of vaccine development. The health agency reports that a total of 30 vaccines are now undergoing human trials, and of these, six candidate vaccines are in the final stage before being approved for public use.
The coronavirus pandemic has now infected more than 23.65 million people and has now killed more than 813,000 people.