The COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected over 33.8 million people worldwide, claiming over one million lives. To date, thousands of pregnant women have been infected with this virus. However, cases of transmission of the infection from the infected mother to the baby are rare and to date, there have been almost no reports of deaths in the neonates infected with the virus.
Researchers from Canada and Brazil collaborated to explore the possibility of perinatal transmission of the virus from pregnant mothers to their babies and the risk of preterm birth. Their study titled, “SARS-CoV-2 cell entry gene ACE2 expression in immune cells that infiltrate the placenta in infection-associated preterm birth,” was published online pre-print and before peer review at medRxiv*.
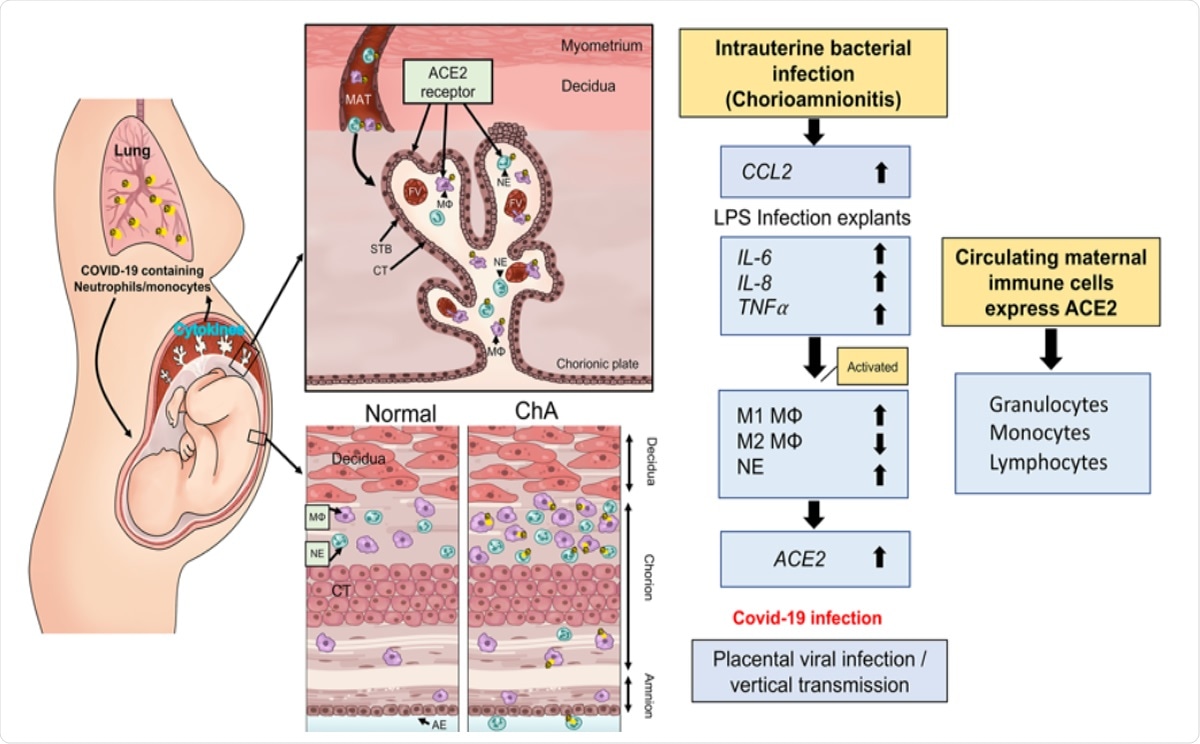
Schematic model by which maternal immune cells could bring SARS-CoV-2 to the placenta and increase risk of vertical transmission in pregnancies complicated by intrauterine infection. Intrauterine bacterial infection, such as occurs in chorioamnionitis (ChA), leads to increased expression and release of chemokine/cytokines by the placenta that in turn induce activation of maternal immune cells, notably M1 macrophage and neutrophils. These cells then target the sites of infection in the placenta/fetal membranes. Subsets of these immune cells express the SARS-CoV-2 cell entry protein, ACE2, and thus are targets for viral uptake in women infected with SARS-CoV-2. The virus may then be hypothetically transported to the placenta increasing the potential for vertical transmission of the virus to the fetus. MAT= maternal blood, FV= fetal vessel, MΦ= M1 macrophage, NE= neutrophil, STB= syncytiotrophoblast, layer CT= cytrophoblast.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
What was this study about?
At present, there are only a handful of reports of transmission of the infection from the mother to baby or vertical transmission of COVID-19. The authors explained that some studies have shown that there is a possibility of infiltration of inflammatory content in the placenta intervillous region. This infiltrate contains neutrophils and monocyte macrophages among pregnant women with SARS-CoV-2 infection. These cells express activation markers. Studies have also shown the potential for transmission of SARS-CoV-2 from an infected mother to her fetus/neonate before birth.
There is little information regarding the risk of complications in pregnancy raised due to COVID-19. However, some reports have shown a higher risk of maternal and neonatal complications in COVID-19 positive mothers. The risk of preterm births, for example, rose to 20 percent, and infection in the neonates was around 6 percent.
The immune system and the placenta
The team writes that the immune system stands between the mother and the fetus, and when the immune system of the mother is disrupted, as in the case of bacterial infection, both the mother and the baby are at risk of other infections, such as viruses. Immune cells that lie between the fetus and the mother thus play a critical role in a successful pregnancy. At the initial stage of the pregnancy, it remodels the uteroplacental circulation, for example, and in the third trimester, it leads to the inflow of maternal peripheral monocytes and immune cells into the uterus that leads to the initiation of the delivery process and labor.
SARS-CoV-2 and pregnancy
SARS-CoV-2 enters the cells through angiotensin-converting enzyme 2 (ACE2). The SARS-CoV spike protein interacts with the host receptor ACE2 using the subunit S1 and then fuses the viral and host membranes through subunit S2.
The fetal heart, kidneys, liver, lung, and placenta contains the ACE2 receptors, and the levels of ACE2 is high in the syncytiotrophoblast layer (STB) and villous stroma between the fetus and the mother.
Hypothesis – infection during pregnancy and risk of COVID-19 transmission
The team hypothesized that if the mother had an infection, her maternal macrophage and neutrophils would be activated, and they would target the uterus. If the ACE2 is expressed on these cells then they have the potential to transport the SARS-CoV-2 virus to the placenta and thus raise the risk of placental infection followed by COVID-19 in the baby.
What was done in the study?
For this study, the team collected information from the placentas from pregnancies that had complications such as chorioamnionitis (ChA). They noted that these placentas had a high expression of ACE2 mRNA.
What was found?
They collected second-trimester placentas and treated them with LPS led to an acute increase in cytokine expression followed by ACE2 mRNA. All the ACE2 protein in the placenta was collected in the syncytiotrophoblast, in fetal blood vessels. There was a rise in M1/M2 macrophage and neutrophils in the villous stroma of the placenta.
M1 macrophage and neutrophils rose in numbers in the placenta of ChA pregnancies. Maternal peripheral immune cells, including granulocytes and monocytes, were found to contain ACE2 mRNA and protein.
Conclusions and implications
The team writes, “our data show that the presence of an intrauterine bacterial infection results in the infiltration of ACE2 expressing maternal macrophage and neutrophils into and across the placental tissues.” The study authors concluded that COVID19 positive pregnancies, especially those with chorioamnionitis, had ACE2 positive immune cells. They wrote, “These ACE2 expressing immune cells have the potential to transport the virus to the placenta in cases of COVID-19 infection in pregnancy and increase the risk of placental infection and vertical transmission of the virus to the fetus.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
SARS-CoV-2 cell entry gene ACE2 expression in immune cells that infiltrate the placenta in infection-associated preterm birth Phatcharawan Lye, Caroline Dunk, Jianhong Zhang, Yanxing Wei, Jittanan Nakpu, Hirotaka Hamada, Guinever Imperio, Enrrico Bloise, Stephen M Matthews, Stephen James Lye medRxiv 2020.09.27.20201590; doi: https://doi.org/10.1101/2020.09.27.20201590, https://www.medrxiv.org/content/10.1101/2020.09.27.20201590v2
- Peer reviewed and published scientific report.
Lye, Phetcharawan, Caroline E. Dunk, Jianhong Zhang, Yanxing Wei, Jittanan Nakpu, Hirotaka Hamada, Guinever E. Imperio, Enrrico Bloise, Stephen G. Matthews, and Stephen J. Lye. 2021. “ACE2 Is Expressed in Immune Cells That Infiltrate the Placenta in Infection-Associated Preterm Birth.” Cells 10 (7): 1724. https://doi.org/10.3390/cells10071724. https://www.mdpi.com/2073-4409/10/7/1724.