The rapidly increasing rate of cases and hospitalizations due to COVID-19, which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), spurred an extensive search for specific and/or effective remedies. One example included the use of COVID-19 CP to prevent the development of severe disease early on in the infection.
The reasons for this interest ranged from the historical effectiveness of this therapy in many infectious diseases, such as the earlier SARS-like pandemics and the H1N1 flu pandemic of 2009. Additional advantages associated with CP include its relative abundance and inexpensive nature. The antibodies in CP are also likely to neutralize newer variants of the virus as they emerge since the plasma will often originate from more recent COVID-19 survivors.
Previous studies indicated a major role of CP in the prevention and treatment of COVID-19. However, later randomized clinical trials failed to confirm the therapeutic benefits of this intervention. In fact, the only benefits reported following the administration of CP to COVID-19 patients occurred when given very early and when CP with a high antiviral neutralizing titer was used.
The early Emergency Use Authorization (EUA) by the Food and Drug Administration (FDA) was therefore revised to high-titer CP, which is defined as CP with a neutralization titer greater than or equal to 250. An additional aspect of this revision included that CP should only be given to hospitalized patients who have early disease, or for immunocompromised patients who cannot generate an adequate humoral response to SARS-CoV-2.
Obstacles to implementing CP use under these conditions include the difficulty of obtaining high-titer plasma, which occurs in only a fraction of infections, as well as the narrow timeframe in which CP can be collected before antibody titers wane. The dilution of the CP antibodies in the recipient’s circulation also posed a concern for its practical use.
Conversely, highly potent neutralizing monoclonal antibodies are being developed for wider use. These are expensive but also effective in reducing disease progression. For this reason, the current study aimed to explore the role of CP in this situation, if not in COVID-19, at least for future pandemics.
About the study
Non-human primates were used to model the human disease, including high viral replication, the immune response to the virus, and the occurrence of interstitial pneumonia. The scientists used pooled CP with a very high neutralizing antibody titer to treat rhesus monkeys at one day post-infection.
CP used in the current study originated from three donors with high-titer antibodies and high anti-spike immunoglobulin G (IgG) antibodies. A pooled seronegative control plasma was also used. Following the completion of a microarray neutralization assay, antibodies were detected against the antigens of most SARS-CoV-2 antigens; however, they showed low reactivity to other coronaviruses.
The macaques were inoculated with SARS-CoV-2 both intranasally and intratracheally, followed one day later with approximately 5 ml/kg of the CP in eight animals, and control plasma in four.
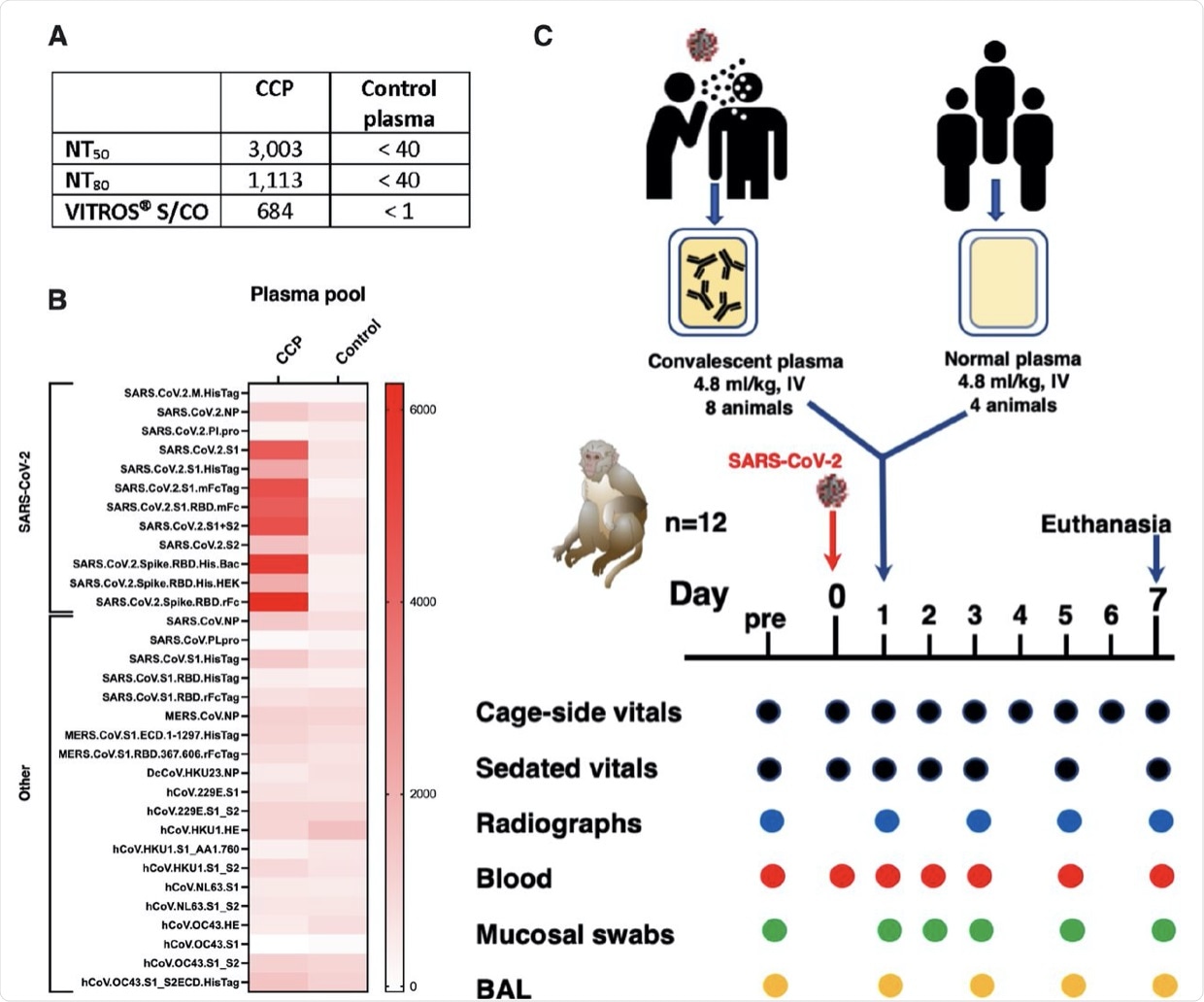 Two pools of human plasma, COVID convalescent plasma (CCP) and normal plasma, were prepared by mixing plasma of convalescent patients or pre-pandemic uninfected donors, respectively. (A) The 2 plasma pools were characterized for neutralizing antibody titers (NT50 and N80 values), and for total spike Ig (by VITROS® assay. (B) The two plasma pools were also tested by coronavirus microarray assay (COVAM), and signal values are graphed as a heatmap. While the CCP had high reactivity to most SARS-CoV-2 antigens, cross-reactivity of the normal plasma pool to SARS-CoV-2 antigens was very low. Both plasma pools had similar reactivity to non-SARS-CoV-2 antigens. (C) Twelve adult rhesus macaques were inoculated on day 0 with SARS-CoV-2 by both intratracheal and intranasal routes. On day 1, eight animals received a single intravenous infusion with pooled CCP, while the other 4 animals received pooled normal control plasma. Animals were monitored closely for clinical signs (both cage-side and sedated observations) with regular collection of radiographs and samples to monitor infection and disease. On day 7, animals were euthanized for detailed tissue collection and analysis.
Two pools of human plasma, COVID convalescent plasma (CCP) and normal plasma, were prepared by mixing plasma of convalescent patients or pre-pandemic uninfected donors, respectively. (A) The 2 plasma pools were characterized for neutralizing antibody titers (NT50 and N80 values), and for total spike Ig (by VITROS® assay. (B) The two plasma pools were also tested by coronavirus microarray assay (COVAM), and signal values are graphed as a heatmap. While the CCP had high reactivity to most SARS-CoV-2 antigens, cross-reactivity of the normal plasma pool to SARS-CoV-2 antigens was very low. Both plasma pools had similar reactivity to non-SARS-CoV-2 antigens. (C) Twelve adult rhesus macaques were inoculated on day 0 with SARS-CoV-2 by both intratracheal and intranasal routes. On day 1, eight animals received a single intravenous infusion with pooled CCP, while the other 4 animals received pooled normal control plasma. Animals were monitored closely for clinical signs (both cage-side and sedated observations) with regular collection of radiographs and samples to monitor infection and disease. On day 7, animals were euthanized for detailed tissue collection and analysis.
Study findings
One day after receiving the CP treatment, all treated animals showed detectable levels of neutralizing antibodies in their serum, which peaked between days 2-5. Some animals showed an enhanced neutralizing response by day 7; however, this is likely due to the host immune response, rather than the CP.
Anti-spike antibodies were also present at one day after infusion, with reactivity to a number of viral antigens that remained detectable throughout the period of observation.
All animals showed only mild-to-moderate disease features, with many being asymptomatic. A runny nose was the most common sign. There was not much difference between the CP-treated and control animals, thereby reducing the usefulness of clinical and radiological markers to determine the therapeutic efficacy of CP.
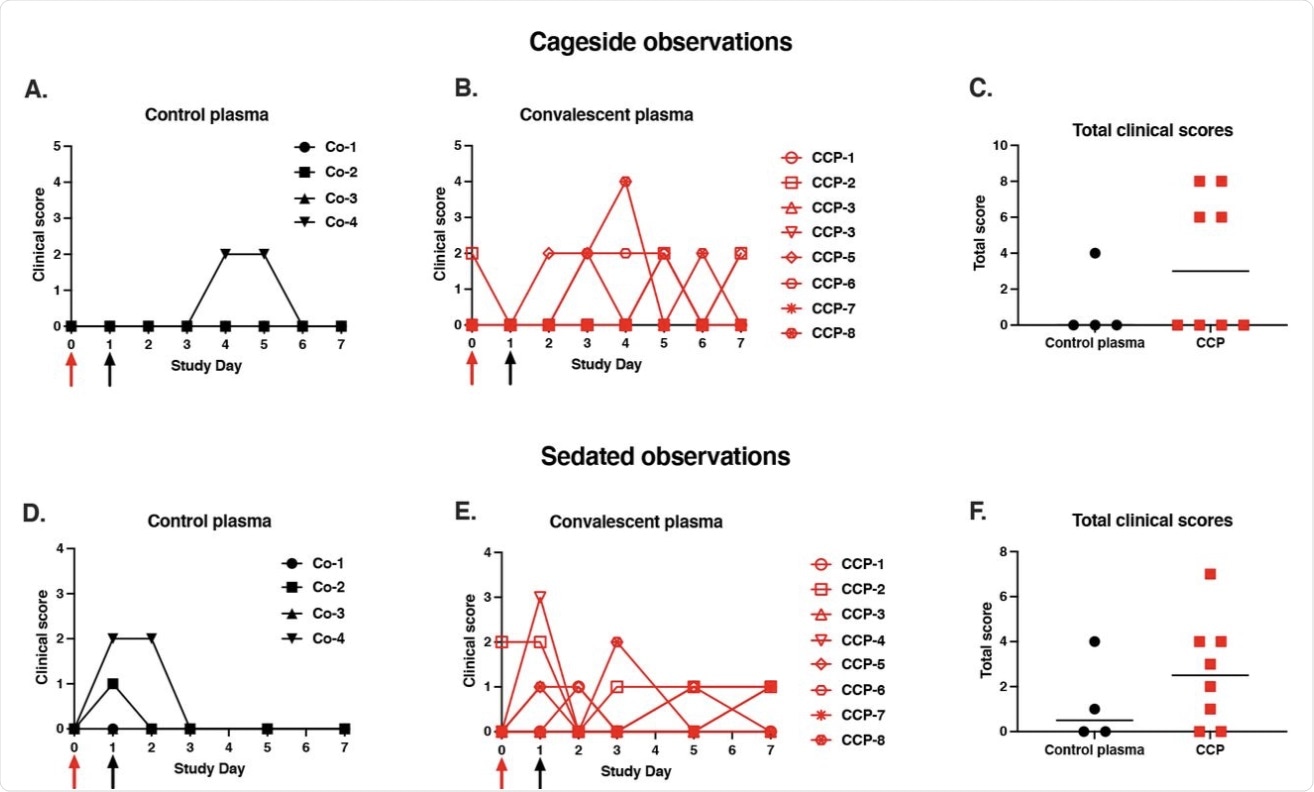 Mild clinical disease course with no detectable effect of convalescent plasma. Red and black arrows indicate time of virus inoculation and plasma administration on days 0 and 1, respectively. (A, B, D, E) Daily clinical scores based on cage-side observations and sedated measurements for each animal of the 2 study groups; the maximum daily score possible is 22 (for cage-side observations) and 27 (for sedated observations). (C, F) For each animal, the total of clinical scores over the 7-day period was tabulated. Comparison of the 2 groups revealed no detectable therapeutic benefits of the CCP treatment (p ≥ 0.28, Mann-Whitney).
Mild clinical disease course with no detectable effect of convalescent plasma. Red and black arrows indicate time of virus inoculation and plasma administration on days 0 and 1, respectively. (A, B, D, E) Daily clinical scores based on cage-side observations and sedated measurements for each animal of the 2 study groups; the maximum daily score possible is 22 (for cage-side observations) and 27 (for sedated observations). (C, F) For each animal, the total of clinical scores over the 7-day period was tabulated. Comparison of the 2 groups revealed no detectable therapeutic benefits of the CCP treatment (p ≥ 0.28, Mann-Whitney).
However, there were signs of activation of both innate and adaptive immunity in both control and treatment groups. This included innate inflammatory cells like neutrophils and pro-inflammatory monocytes and reduced naïve CD4+ T-cells, but not CD8+ T-cells.
Central memory cells increased, whereas effector memory cells decreased as T-cells were activated by the antigen exposure and migrated into the tissues.
The researchers found that viral replication continued unabated within the respiratory tract, as shown by unchanged subgenomic ribonucleic acid (sgRNA) levels in the upper and lower airways. Conversely, lung inflammation in the CP-treated group was markedly reduced by a mean of 17% compared to the control group.
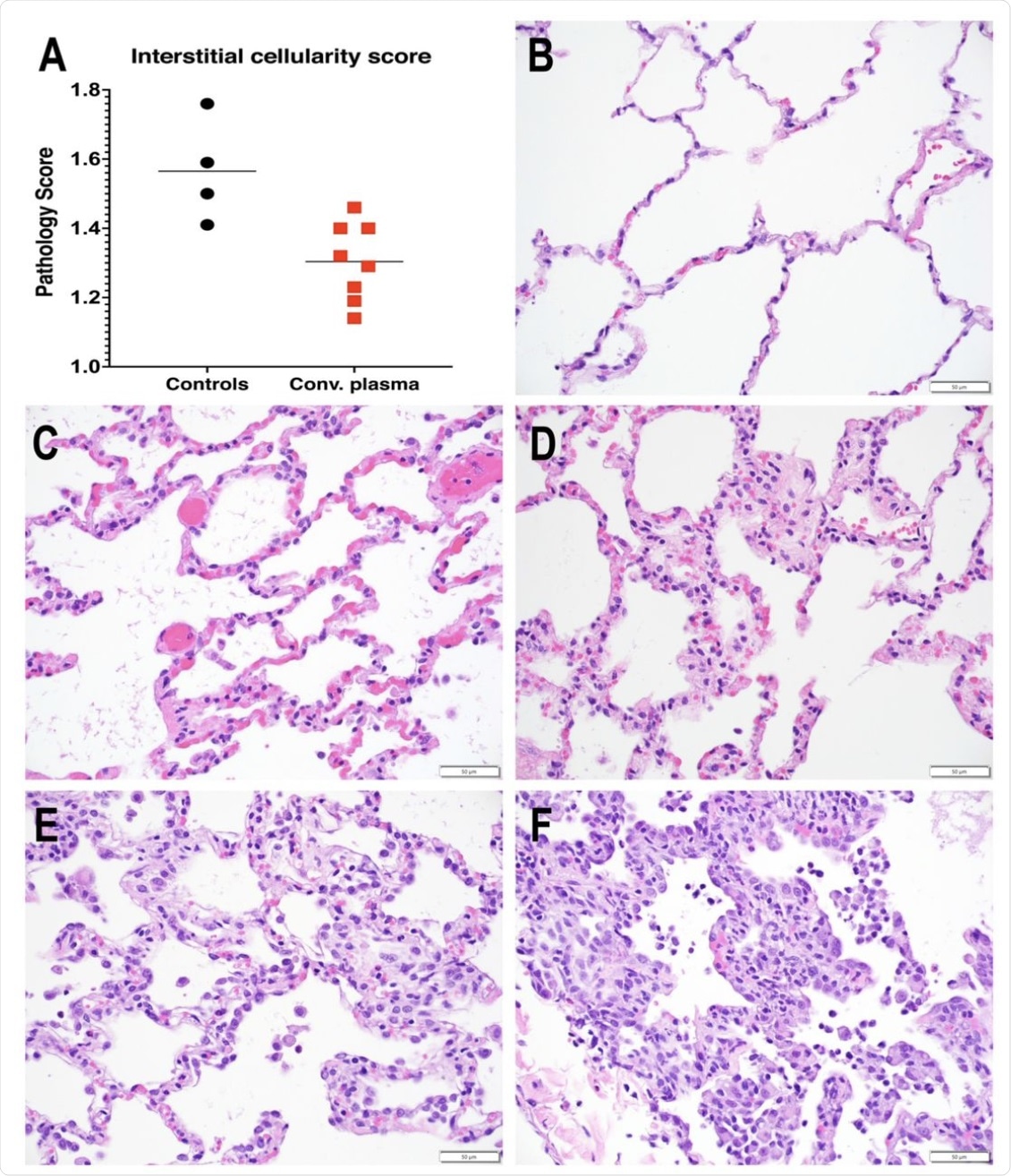 Reduced interstitial pneumonia in convalescent plasma-treated animals. A. Interstitial cellularity was evaluated on 7 lung lobes and an average score was tabulated as outlined in the methods section. Lines indicated mean values. The CCP group had significantly lower scores than the control group (p=0.006; unpaired t-test). B-F. Interstitial cellularity score assigned to random x40 fields is based on the number of cells expanding the alveolar interstitium. Representative x 40 images are shown. B. Grade 0: normal lung with thin acellular alveolar septae (animal CCP-7). C. Grade 1: alveolar interstitium expanded by 1 to 2 cells (animal CCP-1). D. Grade 2: alveolar interstitium expanded by 2 to 4 cells (animal CCP-1) E. Grade 3: alveolar interstitium expanded by 4 to 6 cells. (animal Co-3) F. Grade 4: alveolar interstitium expanded by more than 6 cells (animal Co-3)
Reduced interstitial pneumonia in convalescent plasma-treated animals. A. Interstitial cellularity was evaluated on 7 lung lobes and an average score was tabulated as outlined in the methods section. Lines indicated mean values. The CCP group had significantly lower scores than the control group (p=0.006; unpaired t-test). B-F. Interstitial cellularity score assigned to random x40 fields is based on the number of cells expanding the alveolar interstitium. Representative x 40 images are shown. B. Grade 0: normal lung with thin acellular alveolar septae (animal CCP-7). C. Grade 1: alveolar interstitium expanded by 1 to 2 cells (animal CCP-1). D. Grade 2: alveolar interstitium expanded by 2 to 4 cells (animal CCP-1) E. Grade 3: alveolar interstitium expanded by 4 to 6 cells. (animal Co-3) F. Grade 4: alveolar interstitium expanded by more than 6 cells (animal Co-3)
Implications
The current study demonstrates the efficacy of CP in the treatment of SARS-CoV-2 infections as it prevented viral-induced lung inflammation in a macaque model. The human CP with a high titer of neutralizing and spike-binding antibodies, when given to patients one day after virus inoculation, was beneficial in preventing interstitial pneumonia, but could not reduce viral replication.
CP treatment also failed to show the capacity to reduce viral RNA levels in the respiratory mucosa, which could be explained by the high dilution of the antibodies in the bloodstream following infusion. Therefore, by the time the antibodies reached the mucosal sites, their concentration was too low to impact viral replication.
The animals used in this study were inoculated with a very high dose of SARS-CoV-2, thereby indicating that the CP would have to have very high efficacy to arrest viral replication. This is more so in view of the lag time following CP infusion, during which the antibodies are distributed throughout the body to reach their highest concentration at mucosal sites.
This delay following CP infusion may have been responsible for the lack of any observable effect on viral replication. Moreover, the concentrations of viral particles in the mucosal samples of infected animals were highly variable, thus making it more difficult to detect a significant effect.
The reduction in lung inflammation is a beneficial change, despite the lack of mucosal effects. This is likely because antibody levels required to prevent viral replication in the nasal mucosa are much higher than those that must be present in the lungs for a similar outcome.
“This can explain recent observations that some vaccinated people with breakthrough infections with the SARS-CoV-2 delta variant can have similar viral loads in upper respiratory tract as unvaccinated people, but yet, remain at much reduced risk for severe illness and hospitalization.”
When compared with another study that used monoclonal antibodies, it is clear that the latter prevent lung disease much more effectively in early COVID-19 as compared to when CP was administered. Despite the hurdles to obtaining a constant source of high-dose CP, the investigators suggest that vaccinated people with a history of COVID-19 are the best sources of CP.
Meanwhile, the development of macaque models and assessment scales is likely to be useful in accelerating the testing of antiviral strategies. This could provide information to design future clinical studies as well.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Van Rompay, K. K. A., Olstad, K. J., Sammak, R. J., et al. (2021). Early Post-Infection Treatment Of SARS-Cov-2 Infected Macaques With Human Convalescent Plasma With High Neutralizing Activity Reduces Lung Inflammation. bioRxiv. doi:10.1101/2021.09.01.458520. https://www.biorxiv.org/content/10.1101/2021.09.01.458520v1
- Peer reviewed and published scientific report.
Van Rompay, Koen K. A., Katherine J. Olstad, Rebecca L. Sammak, Joseph Dutra, Jennifer K. Watanabe, Jodie L. Usachenko, Ramya Immareddy, et al. 2022. “Early Post-Infection Treatment of SARS-CoV-2 Infected Macaques with Human Convalescent Plasma with High Neutralizing Activity Had No Antiviral Effects but Moderately Reduced Lung Inflammation.” Edited by Vincent Munster. PLOS Pathogens 18 (4): e1009925. https://doi.org/10.1371/journal.ppat.1009925. https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1009925.