Cancer is a highly complex disease that tends to be caused by the overgrowth of malignant cells and altered immune response. Traditional cancer therapies include surgery, radiotherapy, chemotherapy, and targeted therapy, but none of these can cure cancer entirely.
Immuno-oncology is relatively new in the world of cancer therapy and can control and kill tumor cells by instigating or restoring the immune system.
There are several forms of cancer immunotherapy currently available, including immune checkpoint inhibitors, cancer vaccines, oncolytic virus therapy, T-cell therapy, and monoclonal antibodies. A combination of these effective therapies can greatly enhance efficacy, resulting in a durable antitumor immune response.1
The following article discusses common immunotherapy approaches used in clinical practice and analyzes current challenges in this field.
History of immuno-oncology
After over a century of development, immuno-oncology has emerged as one of the most effective approaches to cancer treatment. In the late 1890s, William Coley conducted pioneering research into immuno-oncology, demonstrating its potential by successfully using bacterial injections to treat cancer.2
During the 1980s, novel cancer treatments began to emerge, including adoptive cell therapy, targeted antibodies, and cancer vaccines.
In 1989, the inception of the first chimeric antigen receptor (CAR) marked a significant breakthrough in medical science. Nearly two decades later, in 2012, Carl June employed CAR-T cell therapy to successfully treat a patient afflicted with leukemia. In the 1990s, PD-1/L1 inhibitors were discovered, and these are now the most widely applied type of immunotherapy.
In recent times, immune checkpoint inhibitors like ipilimumab, nivolumab, and atezolizumab have received approval from the Food and Drug Administration (FDA) for the treatment of cancer.3,4
Types of cancer immunotherapy
Immunotherapy has shown great potential in cancer treatment, with promising approaches found in adoptive cell therapies (ACT), bispecific T cell engagers, immune checkpoint inhibitors, and therapeutic cancer vaccines, for example. 5
These approaches all share a mechanism of action, namely in protecting against tumor antigens by stimulating the T cell-based immune response (Figure 1).
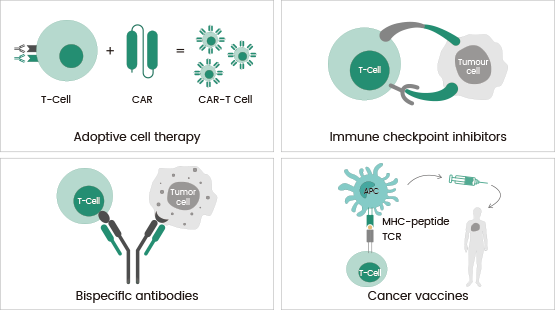
Figure 1. Major types of immunotherapy. Image Credit: Sino Biological US Inc.
Adoptive cell therapy
Adoptive cell therapy is a passive method of immunotherapy. There are four major models of adoptive cell therapy, including tumor-infiltrating lymphocyte (TIL) therapy, chimeric antigen receptor (CAR) T cell therapy, engineered T cell receptor (TCR) therapy, and natural killer (NK) cell therapy.
TIL therapy isolates the patient’s immune cells directly, expanding their numbers and then infusing them back into the patient. For TCR and CAR-T cell therapies, immune cells from the patient are isolated and genetically engineered to recognize and hone in on tumor antigens before infusing them back into the patients. 6
Immune checkpoint inhibitors
Immune checkpoints are protective factors found in the immune system that prevent T cell overactivity, which can result in autoimmune damage. However, cancer cells are commonly seen using these checkpoints to evade immunosurveillance.
To address this challenge, scientists developed immune checkpoint inhibitors (ICIs) to disrupt inhibitory signaling pathways. This approach helps restore T cell immune surveillance and kills tumor cells.7
The most widely used targets for ICIs are PD-1, PD-L1, and CTLA-4; these have demonstrated great benefit in nonsmall-cell lung cancer (NSCLC), metastatic melanoma, and renal cancers.8
Antibody-drug conjugates
Antibody-drug conjugates (ADCs) use the antigen-specificity of monoclonal antibodies to target and deliver cytotoxic drugs to tumor cells. There were 15 approved ADCs as of October 2023, with over 150 candidates in clinical trials.
Intensive research efforts are underway to investigate the potential of combining ADCs with chemotherapy, molecularly targeted agents, and immunotherapy. In fact, the success of ICIs has garnered interest in exploring immune-stimulating ADC payloads such as STING agonists and TLR agonists.9
Bispecific antibodies
Bispecific antibodies (bsAbs) represent a versatile group of antibodies derived from monoclonal antibodies, designed to bind either two distinct antigens or two separate epitopes on the same antigen.
The majority of bsAbs fall into the category of bispecific T-cell engagers (BiTEs), which are engineered to target both a tumor antigen and an immune-related molecule, thereby redirecting T cells to the specific tumor antigens to eradicate cancer.10
Cancer vaccines
Cancer vaccines are designed to activate the body's immune response against tumor cells by utilizing tumor antigens, ultimately leading to the destruction of cancer cells. These can be either preventive or therapeutic.
Preventive cancer vaccines, such as the HPV and HBV vaccines (the only two that have been FDA-approved), inhibit the development of certain cancers.
Therapeutic cancer vaccines are specifically intended to stimulate the immune system's response against particular tumor antigens, to manage tumor growth or even induce tumor regression. These are mainly divided into four types: genetically engineered vaccines, tumor whole-cell vaccines, dendritic cell vaccines, and protein-peptide vaccines.11
Challenges in immuno-oncology
Immunotherapies such as ICIs and ACT are immunotherapies that have shown promising efficacy in cancer treatment. There are, however, many barriers in immuno-oncology, including immune-related adverse events (irAEs) discovered during treatment, a limited number of reliable and valid biomarkers for the evaluation of clinical efficacy, and the low tumor response rate of single-agent immunotherapy.
That being said, combination cancer immunotherapy can greatly increase efficacy, durability, and response rates.12 For instance, in 2018, the anti-PD-1 antibody pembrolizumab combined with chemotherapy was approved by the FDA as a first-line treatment for NSCLC, significantly improving patient survival versus monotherapy.8
Summary
Despite having a long history, immuno-oncology has experienced significant advancements, primarily in recent decades. In this article, Sino Biological discusses the history and different types of cancer immunotherapy and its current challenges. To improve the therapeutic effect of cancer immunotherapy, a combination of multiple methods is effective.
Biomarkers can also be helpful in the diagnosis, progression, and prediction of cancer. Personalized immunotherapy is highly valuable due to the diverse modulation pathways of anticancer immune responses and patient-specific variations.
Sino Biological provides complete solutions for cancer immunotherapy development, including CAR-NK, CAR-T, and bispecific and multispecific antibodies.
Further to this, Sino Biological has developed a comprehensive collection of 1,100+ cytokines, covering all cytokine families, as well as high-quality immune checkpoint and cancer vaccine-related products, to assist in developing innovative cancer immunotherapies.
Sino Biological has established many advanced technology platforms to provide full-service CRO services. These platforms include mammalian cell expression, hybridoma development, and single B cell discovery, all aimed at enhancing the development and production of innovative therapies.
References and further reading
- Taefehshokr N, Baradaran B, Baghbanzadeh A, Taefehshokr S. Promising approaches in cancer immunotherapy. Immunobiology. 2020;225(2):151875. doi:10.1016/j.imbio.2019.11.010
- Wang DR, Wu XL, Sun YL. Therapeutic targets and biomarkers of tumor immunotherapy: response versus non-response. Signal Transduct Target Ther. 2022;7(1):331. doi:10.1038/s41392-022-01136-2
- Galon J, Bruni D. Tumor Immunology and Tumor Evolution: Intertwined Histories. Immunity. 2020;52(1):55-81. doi:10.1016/j.immuni.2019.12.018
- Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807-821. doi:10.1038/s41423-020-0488-6
- Dagher OK, Schwab RD, Brookens SK, Posey AD Jr. Advances in cancer immunotherapies. Cell. 2023;186(8):1814-1814.e1. doi:10.1016/j.cell.2023.02.039
- Wang Z, Cao YJ. Adoptive Cell Therapy Targeting Neoantigens: A Frontier for Cancer Research. Front Immunol. 2020;11:176. doi:10.3389/fimmu.2020.00176
- Wei G, Zhang H, Zhao H, et al. Emerging immune checkpoints in the tumor microenvironment: Implications for cancer immunotherapy. Cancer Lett. 2021;511:68-76. doi:10.1016/j.canlet.2021.04.021
- Barbari C, Fontaine T, Parajuli P, et al. Immunotherapies and Combination Strategies for Immuno-Oncology. Int J Mol Sci. 2020;21(14):5009. doi:10.3390/ijms21145009
- Janet M. Sasso, Rumiana Tenchov, Robert Bird, et al. The Evolving Landscape of Antibody–Drug Conjugates: In Depth Analysis of Recent Research Progress. Bioconjugate Chemistry 2023 34 (11), 1951-2000. doi: 10.1021/acs.bioconjchem.3c00374
- Esfandiari A, Cassidy S, Webster RM. Bispecific antibodies in oncology. Nat Rev Drug Discov. 2022;21(6):411-412. doi:10.1038/d41573-022-00040-2
- Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21(6):360-378. doi:10.1038/s41568-021-00346-0
- Marshall HT, Djamgoz MBA. Immuno-Oncology: Emerging Targets and Combination Therapies. Front Oncol. 2018;8:315. doi:10.3389/fonc.2018.00315
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.