Antibodies are Y-shaped protective proteins manufactured by B lymphocytes in reaction to invading pathogens. When B lymphocytes encounter foreign antigens expressed by antigen-presenting cells (e.g. macrophages or dendritic cells), they are separated and activated by T helper cells or antigen-coated pathogens.
Mature B lymphocytes emit antigen-specific antibodies or separate them into memory B lymphocytes for long-term protection (see Figure 1).
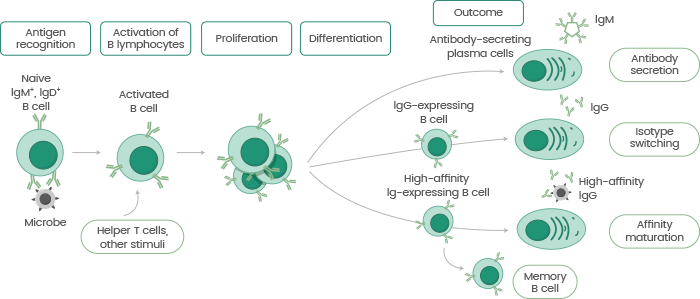
Figure 1. The process of antibody production. Image Credit: Sino Biological Inc.
B lymphocytes are capable of recognizing antigens, and each B lymphocyte may only bind to one type of antigen, signaling its specificity. This particular property demonstrates the possible role of antibodies in therapeutic reagents in modern medicine.
Background
The protective and prophylactic capabilities of antibodies were initially uncovered in the late 19th century, where passive transmission of antibodies from diseased animals offered immunity against diphtheria. Antibodies offer protection against transmitted diseases and can eliminate infection.
Polyclonal antibodies comprise a variety of antibodies that can recognize different antigens. This means they make a significant contribution to research and diagnosis. However, due to limited supply, cross-reactivity, batch-to-batch variation, and lack of specificity, the use of polyclonal antibodies is constrained.
Monoclonal antibodies (mAbs) display specificity to a single epitope. Köhler and Milstein produced the first mAb using hybridoma technology in 1975. This technology utilizes the fusion of antibody-producing cells isolated from spleen tissue of animals that were immunized with immortal myeloma cells (see Figure 2).
The ‘hybridoma’ was born with its potential for producing unlimited amounts of monospecific antibodies, significantly developing the basic research and capability for their clinical use.
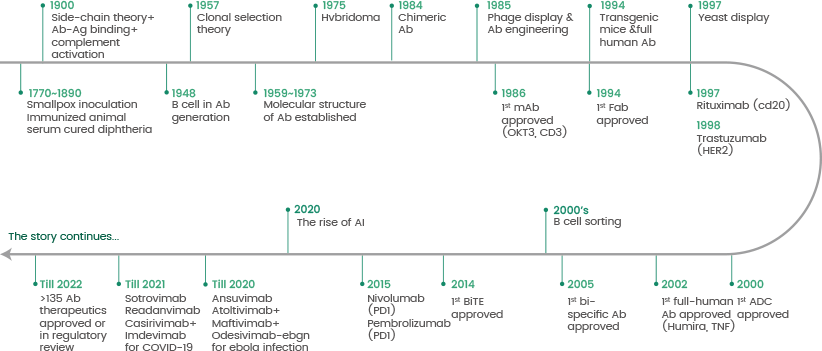
Figure 2. The history of antibody production. Image Credit: Sino Biological Inc.
Despite mAbs showing a promising therapeutic index, the murine protein restricts their therapeutic application in humans because of allotypic immune responses that clear the non-human antibody at a rapid rate.
Developments in genetic engineering technology have pushed antibody manufacture to a new age of humanized antibody production that is appropriate for the therapy of humans.
In humanized antibody production, human immunoglobulin loci are introduced into the germline of transgenic mice to create human antibodies to resolve immune rejection.
The main disadvantages of mAbs are that they are time-consuming and costly to prepare. Humanized mouse models lack critical molecules for robust functional cellular and humoral responses.
An alternative humanized antibody approach is using complementarity-determining region (CDR) grafting, developed in 1986 by Gregory P. Winter. Non-human antibody CDR sequences are transferred into the human framework sequence, enabling mAbs to sustain binding specificity to the target antigen.
Humanized antibodies have generated encouraging results for treating diseases that require long-term treatment, such as cancer and infectious diseases.
Single B cell and phage display technologies have been created to produce mAbs. Phage display is utilized for in vitro mAb selection and the prompt identification of antibody fragments or peptides.
In 1985, George P. Smith utilized recombinant DNA technology to fuse M13 bacterio phage with foreign peptides to show the foreign peptide on the phage surface. Phage display technology has been utilized for in vitro affinity screening to develop monoclonal antibodies.
Single B cell antibody technology enables the rapid and highly efficient isolation of potential mAbs. Single B cell cloning maintains the pairs of light and heavy chains. Single B lymphocytes may be isolated via fluorescence-conjugated antigens to establish antigen specificity (see Figure 3).
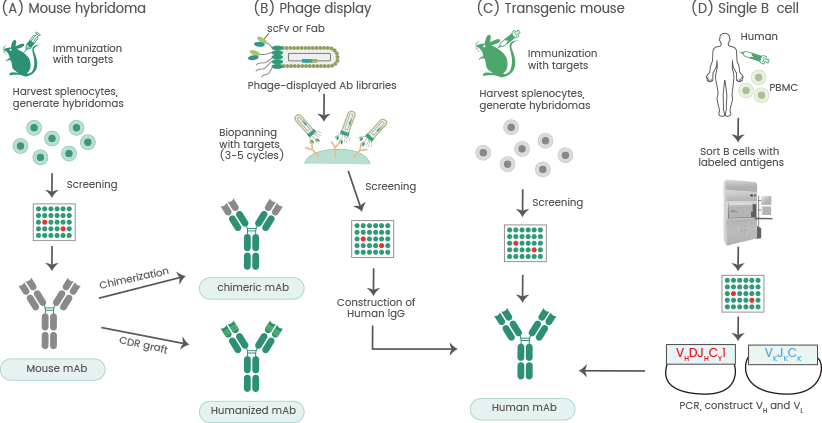
Figure 3. Approaches for the development of therapeutic antibodies. a The traditional mouse hybridoma technique starts by immunization of mice with desired antigens to trigger an immune response. Harvested splenocytes are fused with myeloma cells to produce hybridoma cells that persistently secrete antibodies. After the screening, selected leads are used to generate chimeric or humanized antibodies. b Phage display. A human phage-displayed human antibody library is used to select antigens of interest. After 3–5 rounds of biopanning, immuno-positive phage clones are screened by ELISA; then DNA sequences are analyzed to construct and express human IgGs. c Transgenic mouse. Similar to the mouse hybridoma technique or single B cell methods. d The single B cell technique. From infected or vaccinated donors, PBMCs are prepared for isolation of suitable B cells by flow cytometry. Following the RT-PCR, VH and VL information of each B cell informs the generation of human mAbs. Source: doi.org/10.1186/s12929-019-0592-z. Image Credit: Sino Biological Inc.
As a result of the rapidly growing technologies of gene editing and recombinant proteins, recombinant antibodies do not require immunization and may be created in vitro by multiple organism expression systems.
The intrinsic characteristics, such as pharmacokinetics, binding affinity, immunogenicity, and specificity may also be adapted easily with the use of mutagenesis methods.
Antibody engineering methods can modify the traditional Y-shape of full-length antibodies into a variety of types, such as VHH, scFv, and Fab, which have been created to develop new therapeutic drugs.
Modulating recombinant antibodies can enhance immune responses, such as developing fusion antibodies, improving affinity against conserved targets, engaging immune effector functions, allowing efficient tissue penetration, and increasing the half-life time in circulation.
Since the US Food and Drug Administration (FDA) approved the first therapeutic mAb, muromonab-CD3, mAbs have become the leading therapeutic modality in modern medicine. Presently, a minimum of 579 therapeutic mAbs have been investigated, and 100 therapeutic mAbs have been approved for clinical use.
The first fully human therapeutic antibody based on phage display technology was Adalimumab (Humira), an anti-tumor necrosis factor alpha antibody. It was approved in 2002 for the treatment of rheumatoid arthritis.
In 2006, The FDA approved the first human antibody generated in a transgenic mouse, an anti-epidermal growth factor receptor (EGFR).
At present, half of the treatment-approved mAbs are either humanized or chimeric. Among the most recognized humanized antibodies, Trastuzumab treats human epidermal growth factor receptor 2-positive metastatic breast cancer and gastroesophageal junction adenocarcinoma.
Conclusion
Antibodies are vital molecular sentinels that are highly specified to their corresponding antigens. Their utilization is popular in life science research and as therapeutics. With the fast-growing interest in therapeutic mAbs in clinical studies, more resources are invested in the field.
As a worldwide reagent and services supplier, Sino Biological provides a range of customized services, mostly focused on recombinant production of antibodies and antigens, in addition to ‘TOP 10’ pharmaceutical companies and biotechnology firms pre-clinical production technology services for furthering the development of mAb drug candidates.
References and further reading
- Lu et al. (2020) Journal of Biomedical Science 27, p. 1. doi.org/10.1186/s12929-019-0592-z
- Saeed AFUH, Wang R, Ling S and Wang S (2017) Front. Microbiol. 8, p. 495. doi: 10.3389/fmicb.2017.00495
- Alberts B, Johnson A, Lewis J, et al. (2002) Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. B Cells and Antibodies.
- DeLuca et al. (2021) Generation and diversification of recombinant monoclonal antibodies for studying mitosis. BioRxiv 11 Sep 2021,doi.org/10.1101/2021.09.10.455288
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.