Mpox virus (MPXV,) previously known as monkeypox, is an old virus that has recently garnered a lot of interest due to its emergence in uncommon regions of the world.
Generally endemic to Central and Western Africa, MPXV – which caused the Monkeypox virus disease – was first isolated in 1958 in Copenhagen, Denmark. The first reported case of this virus in a human was in 1970 in Central Africa.
The virus has two distinct clades, the Congo Basin and the West African clade. These two clades occur respectively in Central and Western Africa. The Congo Basin clade causes a more severe infection than the West African clade.
The first cases of MPXV in the US were reported in 2003. At the time, the virus was predicted to become a widespread infection, but it was successfully contained without a significant rise in the number of cases. It was not until 2022 that MPXV spread became a global concern, with rising cases occurring in the Western Hemisphere.
MPXV is a member of the poxviruses, the Orthopoxvirus (OPXV) genus, and the Poxviridae family. A well-known member of the poxvirus family is smallpox. The clinical features of MPX are similar to that of smallpox, which includes rashes, lesions, and pustules, accompanied by flu-like symptoms.
However, unlike smallpox, MPXV is milder and has a low mortality rate. Viral transmission in humans occurs through direct and prolonged contact with fomites, infected animals, or other infected individuals. Many of the 2022 outbreak cases have been linked to intimate physical contact with an infected individual.
Mpox proteins in immunodiagnostics
With the emergence and rise of MPX cases in the US and other non-endemic countries, there is a crucial need for an enhanced understanding of the mechanisms of virus infectivity and virulence.
MPXV is an enveloped, double-stranded DNA virus. Such viruses can exist in two distinct forms: an intracellular mature virus (MV) and an extracellular enveloped virus (EV). It can enter the cell through cell attachment and fusion, or the MV can be released from infected cells via lysis, whereas an EV exits host cells via exocytosis.
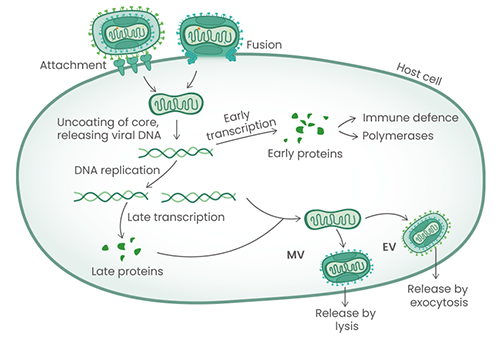
Figure. The MPXV life cycle with the different viral antigens playing role at the indicated steps. Image Credit: Sino Biological Inc.
Some key MPXV proteins are homologous to those expressed by the vaccinia virus (VACV), which is the model poxvirus.
Examples of these proteins include:
- A35R (VACV-A33R)
- A29 (VACV A27L)
- B6R (VACV-PS/HR)
- M1R (VACV-L1R)
- H3L (VACV H3L)
- L1R (VACV J1R)
These proteins are involved in promoting virulence, assisting the virus in attachment and entry into the host cell, viral particle replication, packaging, and inhibition of the host’s immune response (Table 1).
These proteins collectively make viral morphogenesis and subsequent pathogenesis possible. Thus, it is essential to study MPXV in detail to help understand the role of these viral proteins in promoting and causing MPX.
Table 1. Selected Monkeypox proteins and their functions. Source: Sino Biological Inc.
| Viral antigen |
Function |
| L1R |
Cell entry |
| A29 |
Intracellular MV trafficking |
| H3L |
Mediates cell adsorption and Inhibits mammalian immune system |
| I1L |
Production of mature virions (MV) |
| A30 |
Viral entry and biosynthesis |
| A35 |
Cell-to-cell spread of viral particles |
| M1R |
Inhibits mammalian immune system |
| B6R |
Inhibits mammalian immune system |
Current diagnostic approaches for MPXV involve using standard PCR protocols as well as immunodetection of viral proteins or neutralizing antibodies already present in a patient’s blood samples.
While traditional PCR-based tests remain the gold standard, immunodiagnostic methods have the benefits of being more cost-effective, quicker, and easier. The antigen test is an alternative diagnostic method that relies on the immunodetection of viral antigens in biological samples.
The presence of antigens is a direct indication that the individual carries the virus. An enzyme linked immunosorbent assay (ELISA) can be used to serologically detect antibodies that can work against MPXV infection.
New, improved, more sensitive, and specific viral proteins and antibodies against MPXV viral particles are of interest to researchers. Additionally, they are also required to improve current immunodetection techniques.
These research products will aid in the development of an inexpensive, easily and rapidly manufacturable vaccine that will be effective and available worldwide.
Mpox proteins and antigen detection antibodies from Sino Biological
To aid researchers on their journey, Sino Biological offers a variety of MPXV proteins and antibodies to accelerate the development of improved immunodiagnostics and vaccines against MPXV. Previously, the company provided researchers with key solutions to detect and combat the SARS-CoV-2 virus.
At present, the company has sensitive and specific MPXV antibody pairs for ELISA to capture and detect the A29 MPXV protein (Table 2), with western blot applications as well. A collection of purified recombinant MPXV proteins from different expression systems are also available to facilitate MPXV research (Table 3).
Table 2. Monkeypox antibodies for ELISA and western blot analysis. Source: Sino Biological Inc.
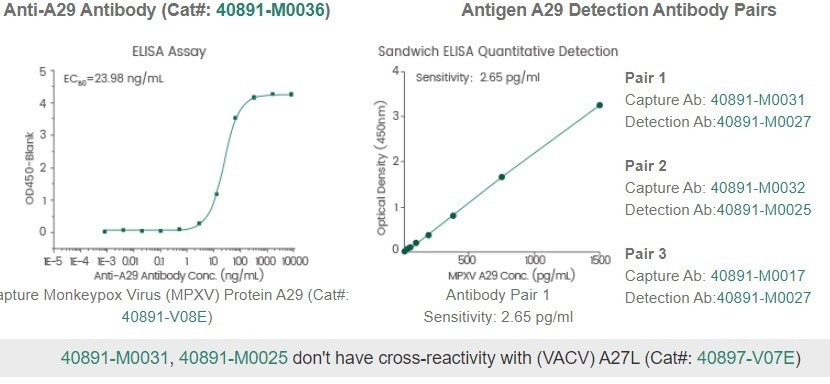
Table 3. Monkeypox proteins available for research purposes. Source: Sino Biological Inc.
| Cat# |
Antigen |
Strain |
Expression Host |
Purity |
Tag |
| 40886-V07E |
A35 |
West African strains (MPXV) |
E. coli |
>90% |
N-His |
| 40902-V08H |
B6R |
West African strains (MPXV) |
HEK293 Cell |
>90% |
C-His |
| 40904-V07H |
M1R |
West African strains (MPXV) |
HEK293 Cell |
>90% |
N-His |
| 40893-V08H1 |
H3L |
West African strains (MPXV) |
HEK293 Cell |
>95% (by SEC-HPLC) |
C-His |
| 40886-V08H |
A35 |
West African strains (MPXV) |
HEK293 Cell |
>90% (by SEC-HPLC) |
C-His |
| 40891-V08E |
A29 |
West African strains (MPXV) |
E. coli |
>90% |
C-His |
| 40889-V07E |
L1R |
West African strains (MPXV) |
E. coli |
>90% |
N-His |
| 40888-V07E |
I1L |
West African strains (MPXV) |
E. coli |
>90% |
N-His |
| 40885-V53E |
A33R |
West African strains (MPXV) |
E. coli |
>85% |
N-GST & His |
| 40896-V07E |
A33R |
strain Copenhagen (VACV) |
E. coli |
>90% |
N-His |
| 40897-V07E |
A27L |
strain Copenhagen (VACV) |
E. coli |
>95% |
N-His |
| 40900-V08H |
PS/HR (B5R) |
strain Copenhagen (VACV) |
HEK293 Cell |
>90% |
C-His |
| 40903-V07H |
L1R |
strain Copenhagen (VACV) |
HEK293 Cell |
>95% |
N-His |
References and Further Reading
- Kumar, N., Acharya, A., Gendelman, H. E., & Byrareddy, S. N. (2022). The 2022 outbreak and the pathobiology of the monkeypox virus. Journal of Autoimmunity, 102855.
- Taub DD, Ershler WB, Janowski M, Artz A, Key ML, McKelvey J, Muller D, Moss B, Ferrucci L, Duffey PL, Longo DL. Immunity from smallpox vaccine persists for decades: a longitudinal study. Am J Med. 2008 Dec;121(12):1058-64. doi: 10.1016/j.amjmed.2008.08.019. PMID: 19028201; PMCID: PMC2610468.
- Weaver JR, Isaacs SN. Monkeypox virus and insights into its immunomodulatory proteins. Immunol Rev. 2008 Oct; 225:96-113. doi: 10.1111/j.1600-065X.2008.00691.x. PMID: 18837778; PMCID: PMC2567051.
- Xiang Y, White A. Monkeypox Virus Emerges from The Shadow of Its More Infamous Cousin: Family Biology Matters. Emerg Microbes Infect. 2022 Jun 24:1-14. doi: 10.1080/22221751.2022.2095309. Epub ahead of print. PMID: 35751396.
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.