A subgroup of the mutations detected in the RBD domain of the spike protein appears in more than one strain. These overlapping mutations generate considerable interest because they could cause increased transmissibility. Sino Biological has developed RBD and Spike proteins, each of which covers these variants.
The mutations in these strains may also be seen in the nucleocapsid protein, which is the biomarker commonly targeted by rapid antigen tests. Therefore, it is of utmost importance to determine whether commercial antigen tests available on the market can detect mutated N proteins with equal sensitivity and specificity as their WT counterpart.
Sino Biological has developed a range of high-grade research reagents for dominant SARS-CoV-2 mutants. These include recombinant antigens, ELISA kits, cDNAs, detection antibodies, peptide pools, and receptor ACE2. Sino Biological’s variant reagents have played an active role in the fundamental research areas of SARS-CoV-2, including the development of therapeutic antibodies, vaccines, and diagnostic reagents.
Advantages of SARS-CoV-2 mutant reagents
Source: Sino Biological Inc.
| . |
. |
| Recombinant Antigens |
- Multiple antigens: RBD, S-NTD, S1, S-ECD, S-ECD-trimer, Nucleocapsid
- Wide coverage of variants: >100 types, including latest subvariants JN.1, BA.2.86, EG.5.1
- Various tags: His, mFc, Fc, rFc, His-AVI
- 3 expression systems: HEK293, Insect, E. coli
- Elite quality: Validated by SDS-PAGE, SEC-HPLC, SEC-MALS, ELISA, BLI, etc.
|
| Detection Antibodies |
- Various applications: Neutralization, WB, ELISA, IHC, IF, FCM, IP, LFA (lateral flow immunoassay), etc.
- Multiple epitopes: RBD (competition, non-competition), S-NTD, S2, N-NTD, N-CTD, and Nucleocapsid (disordered area)
- Elite quality: High affinity and specificity validated by SPR, ELISA, and WB
|
| ELISA Kits |
- Comprehensive types: Inhibitor screening kits, antibody titer assay kits, and antigen detection kits
- Wide applications: Apply for the detection of RBD/S1/NP from wild type, Omicron, and VOC strains
- Rigorous QC standard: Test of linearity, cross-reactivity, sensitivity, specificity stability, and precision
- Fast response: 100+ antibodies reserved, ensuring rapid development of ELISA kit by screening antibody pairs that recognize new mutants
|
| More Products |
Peptide Pools
- Multiple categories: Sipke, S1, S2, S-NTD, RBD, RBD-RBM, RBD-Non RBM
cDNAs
- 2 expression vectors: pCMV3 vector, lentiviral vector
- Multiple tags: Myc, HA, FLAG, His, GFPSpark®, OFPSpark®
- Quality assurance: Full length sequence confirmed
ACE2
- Validated by ELISA: ACE2 protein can bind to spike
|
Recommended recombinant SARS-CoV-2 variants
Latest mutants
Omicron (JN.1) spike S1+S2 trimer protein (ECD, his tag)
Cat#: 40589-V08H59
HPLC & SEC-MALS-verified
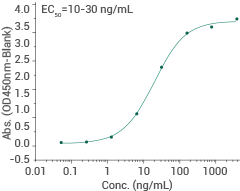
Immobilized recombinant human ACE2 (Cat#: 10108-H05H) can bind JN.1 Spike S1+S2 trimer Protein. Image Credit: Sino Biological Inc.
Omicron (BA.2.86) spike RBD protein (aa319-529)
Cat#: 40592-V08H152
HPLC-verified
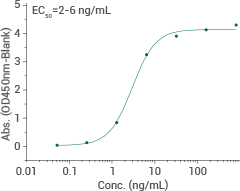
Immobilized recombinant human ACE2 (Cat#: 10108-H05H) can bind BA.2.86 spike RBD protein. Image Credit: Sino Biological Inc.
Omicron (EG.5.1) spike S1+S2 trimer protein (ECD)
Cat#: 40589-V08H55
HPLC & SEC-MALS-verified
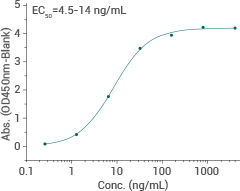
Immobilized recombinant human ACE2 (Cat#: 10108-H05H) can bind EG.5.1 spike S1+S2 trimer protein. Image Credit: Sino Biological Inc.
Applications
Basic research
Featured products
Various Variants: Alpha B.1.1.7 (Cat#: 40589-V08H12), Beta B.1.351 (Cat#: 40589-V08H13), Delta B.1.617.2 (Cat#: 40589-V08H10), Kappa B.1.617.1 (Cat#: 40589-V08H11) Omicron EG.5.1 (Cat#: 40589-V08H55), Omicron FY.4 (Cat#: 40589-V08H53).
Trimer purity: >95% validated by SEC-MALS
Binding to ACE2 verified by ELISA
Covering dominant omicron strains: Omicron BA.2.86 (Cat#: 40592-V08H152), Omicron EG.5.1 (Cat#: 40592-V08H151, 40589-V08H55)
Specific antibodies: Pseudovirus assays (BA.4 Spike PSV (Cat#: PSV022) and BA.5 Spike PSV (Cat#: PSV023)) used to validate high-neutralization activity (Cat#: 40589-D003).
Vaccine development
Featured products
300+ recombinant SARS-CoV-2 variants: These address a variety of strains, several antigens, and a diverse range of tags.
Neutralizing antibodies, specific antibodies, and broad-spectrum antibodies: Specificity and neutralization activity verified by ELISA and/or PSV assay.
SARS-CoV-2 variants detection antibodies: Batches of antibodies to combat SARS-CoV-2 mutant antigens, including antibody pairs, neutralizing antibodies, and specific antibodies.
Peptide pools: Various full-length spike, S1, S2, S-NTD, RBD, RBD-RBM, RBD-Non RBM types.
30+ ELISA Kits: used for antigen detection, inhibitor screening, and antibody titer assay. Kits are comprised of high affinity monoclonal antibodies, high specificity and sensitivity, with scale-up production capacity and lot-to-lot consistency.
Source: Sino Biological Inc.
| Cat# |
Description |
| KIT001 |
SARS-CoV-2 Inhibitor Screening Kit |
| KIT004 |
SARS-CoV-2 Spike S1+S2 ECD Antibody Titer Assay Kit |
| KIT017 |
SARS-CoV-2 Omicron (B.1.1.529) Spike S1+S2 ECD Antibody Titer Assay Kit |
| KIT40592C |
SARS-CoV-2 Omicron (B.1.1.529) Spike RBD Antigen Detection Kit |
| KIT023 |
SARS-CoV-2 (B.1.1.529) Nucleocapsid/N Antibody Titer Assay Kit |
Drug development
Featured products
Diagnostic reagents development
A comprehensive collection of antigens and antibodies for SARS-CoV-2
Used by hundreds of companies for diagnostic kit development
Has been used in manufacturing diagnostic kits worldwide.
ELISA Kit development
Featured products
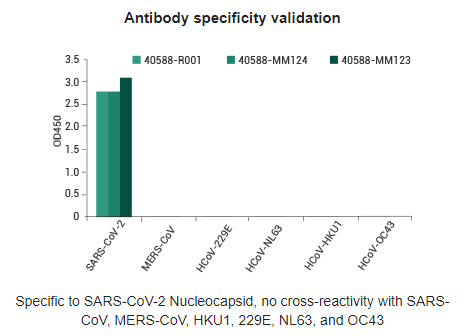
- Antibody pairs for the detection of SARS-CoV-2 N and S protein: Sino Biological has developed a series of antibody pairs against the SARS-CoV-2 Spike and N proteins, both of which bind specifically to the SARS-CoV-2 antigen
- SARS-CoV-2 spike specific antibodies: Specificity verified by ELISA assay
Recommended products and services
- SARS CoV
- MERS CoV
- HCoV-HKU1
- HCoV-NL63
- HCoV-229E
- HCoV-OC43
- DcCoV
- Human Coronavirus Reagents
- Four systems
- Experience with 8,000+ Proteins
Sino Biological can provide custom-made protein services ranging from gene synthesis and vector construction to protein expression and purification. This capacity allows the company to provide complete support for SARS-CoV-2 research. Sino Biological possesses cutting-edge capabilities for HTP expression and mass production. Furthermore, the company employs intensive QC measures, such as SDS-PAGE, SEC-HPLC, FACS, and cell-based assays.
- Mammalian Transient Service
- E. coli Expression Service
- Baculovirus-Insect Service
- Stable Cell Line Service
- In-house expression platforms
- Diverse antibody formats
Sino Biological’s proprietary mammalian cell culture and recombinant expression platforms are seen as the company’s main asset. Customers can choose from a range of packages that address recombinant antibody production services to support the particular requirements of SARS-CoV-2 research.
- High-throughput Antibody Service
- Large-scale Antibody Service
- Antibody Fusion Proteins Service
- scFv/Fab/VHH Production Service
- Chimeric Antibody Production Service
- Bispecific Antibody Production Service
- Five platforms
- 30,000+ antibody experience
With five primary antibody development platforms on offer, Sino Biological can provide diverse antibody development service packages which spans the entire process, from antigen design and preparation to animal immunization and antibody purification, meeting the specific needs of SARS-CoV-2 research.
- Hybridoma Platform
- FACS B Cell Platform
- Rabbit pAb Platform
- Phage Display Platform
- Beacon® B Cell Platform
Viral reagents development capacity
In January 2020, Sino Biological created the main spike protein reagents within 11 days. Reagents of SARS-CoV-2 have assisted a vast number of scientists and researchers in over 70 countries worldwide for COVID-19 research, including the research fundamentals of SARS-CoV-2 and the advancement of immunodiagnostic kit and vaccines.
Sino Biological launched the world’s most extensive recombinant viral antigen collection, ProVir®, comprised of over 1000 products from 90+ virus types/subtypes and 350+ strains. The product range features a broader selection of high-grade recombinant proteins expressed in insect and mammalian cells. These products are tested rigorously for their bioactivity and purity.
Featured viral reagents
- Influenza Virus
- RSV Antigens and Antibodies
- Monkeypox Virus (MPXV)
- Ebola Virus (EBOV)
Since September 18, 2023, around 2,000 papers have been published citing the use of Sino Biological’s SARS-CoV-2 reagents and related services.
Sino Biological honors
Sino Biological is committed to research in virology and infectious disease sectors. It was the first in the world to create SARS-CoV-2 (COVID-19) viral antigens.
The company offers a single solution for researchers and pharmaceutical companies across the globe, which can assist researchers and help them obtain the requisite amount of high-quality bioreagents to increase the speed of discovery and improve human health. Sino Biological was the recipient of the "Supplier Succeeding in SARS-CoV-2 research" award from CiteAb in 2021 and 2022.
About Sino Biological Inc.
Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.