Recently, Pfizer made public a $525 million acquisition of ReViral’s novel therapy for respiratory syncytial virus (RSV). Subsequently, there has been an increased interest in developing RSV therapies on a global scale.
Since the 1960s, scientists have been studying and working on therapies for RSV infection, yet a suitably safe and effective vaccine has yet to be found. Thus, Pfizer’s acquisition has sparked some speculation that could herald a new era of RSV treatment.
What is RSV?
RSV is the primary cause of lower respiratory tract infections in infants and children around the world, with considerably high mortality and morbidity rates. In the senior citizens, RSV reinfections can lead to fevers, pneumonia and even death.
Each year in the USA, there are 58,000 hospitalizations of children under the age of 5 years and 177,000 hospitalizations of older adults aged 65 years due to RSV, according to the Centers for Disease Control and Prevention.
Of all the RSV infection cases in elderly patients, 13,000 were fatal. RSV belongs to the family Paramyxoviridae which is an enveloped negative-strand RNA virus. It is comprised of 10 genes that encode 11 proteins.
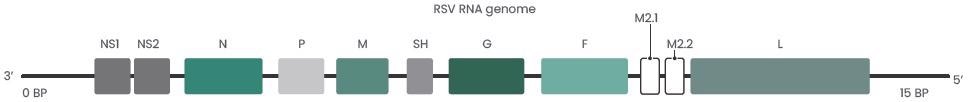
Image Credit: Drysdal, et al. Sci. Transl. Med. 2020
RSV has two primary subtypes: A and B. Although both strains are often in cocirculation, only one largely causes infections in a given period of time.
RSVs generally include various envelope, structural and nonstructural proteins:
- Envelope proteins: G glycoprotein, fusion (F) glycoprotein, and small hydrophobic (SH) protein
- Structural proteins: Large (L) protein, nucleocapsid (N), phosphoprotein (P), matrix (M), M2-1, and M2-2
- Nonstructural proteins: NS1, NS2
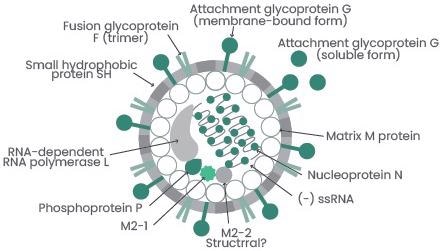
Image Credit: Pablo A. González, et al. Rev. Med. Virol. 2012
The following table shows the most commonly developed targets for drug, vaccine and immunodiagnostic development:
Source: Sino Biological Inc.
| Targets |
Function |
Application |
| Fusion(F) glycoprotein |
Fusion and cell entry; Highly conservative |
Vaccine, drugs, and immunodiagnosis |
| G glycoprotein |
Host cell attachment; Highly Variable |
Vaccine |
| Nucleocapsid (N) |
Envelop virus genome |
Vaccine, immunodiagnosis |
How can RSV reagents be applied in research?
Vaccine development
While there are more than 60 RSV vaccine programs around the world in development today, no approval has been granted for clinical use for any given vaccine. During the development process, researchers have employed extensive knowledge of viral vaccines. For instance, most vaccines target the fusion (F) glycoprotein.
However, G glycoprotein is more rarely used in RSV vaccine research because it possesses characteristics that are deemed highly variable.
Sino Biological’s RSV reagents have supported preclinical studies, clinical trials, and quality control sessions throughout the various development phases of the RSV vaccine, including vaccine content testing, vaccine efficacy testing, and toxicology research.
Smith et al. (2017) applied recombinant RSV-F protein (Cat#: 11049-V08B) to identify serum antibody titers following vaccination in the study of the DNA vaccine delivery mechanism in a previous investigation of DNA vaccine delivery mechanisms.
The data outlines the findings of a preclinical study evaluating the safety and efficacy of a vaccine delivery system (Smith, et al. Vaccine. 2017). In a similar manner, another study applied recombinant RSV-G protein (Cat#: 40830-V08H) to identify RSV A2 virus-specific antibodies in serum.
Therapeutic drug development
AstraZeneca’s Synagis® (Palivizumab) is the only globally approved drug for the prevention of RSV infection. It is a humanized murine monoclonal antibody that inhibits the spread of the virus to the lower respiratory tract via deactivation of the RSV fusion glycoprotein.
It is indicated only for high-risk preterm infants and not for postinfection treatment. Numerous monoclonal antibodies and small molecule drugs are still being investigated to develop treatments for RSV infection.
In a previous study that focused on the production of immunoglobulins (Igs) that met the specific therapeutic needs in the treatment of infectious, Jacque et al. prepared affinity chromatography media for anti-RSV immunoglobulin enrichment using recombinant RSV F protein (Cat#: 11049-V08B).
They used a rabbit monoclonal antibody (Cat#: 11049-R009) in the antiviral assay to detect the level of F antigen expressed by RSV infected cells following incubation with anti-RSV Ig. The level of F antigen expression reflected the neutralizing efficiency of anti-RSV Ig. (Jacque et al. Front. Immunol. 2021).
Development of immunodiagnostics
Rapid antigen testing is advantageous as its sensitivity and specificity increase as antigen detection technology progresses.
The development of Quidel’s SofiaTM assay platform reportedly demonstrated sensitivity and specificity of 78.6% and 93.9 %, respectively, while the BD’s VeritorTM assay system demonstrated a sensitivity and specificity of 81.6 % and 99.1 %, respectively (Cameron Griffiths et al. Clin. Microbiol. Rev. 2017).
Sino Biological’s recombinant RSV N and RSV-F proteins are being employed today in the preparation and screening of core raw materials (antibody pairs) for RSV antigen detection kits and quality control samples.
Sino Biological has screened a pair of antibodies that identify fusion glycoprotein from a range of different RSV strains and can be applied as raw materials for RSV detection (Capture antibody, Cat#: 11049-R338; Detection antibody, Cat#: 11049-R302).
Reagent toolkit for RSV
Sino Biological has overseen the development of an extensive collection of RSV-related reagents for viral research (7 antibodies, 17 antigens, 80+ ELISA Kits, and cDNAs) that encompass both RSV A and B from over five strains.
Recombinant RSV antigens
Image Credit: Sino Biological Inc.
Anti-RSV antibodies
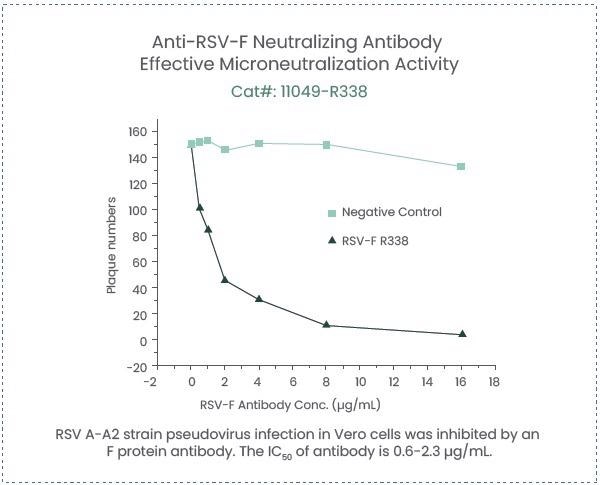
Image Credit: Sino Biological Inc.
Anti-RSV-F rabbit monoclonal antibody
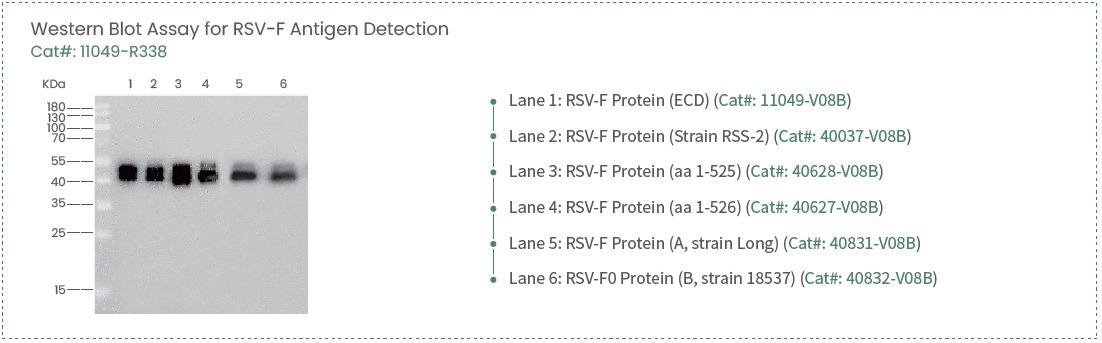
Image Credit: Sino Biological Inc.
To discover more about RSV related reagents, please visit: https://www.sinobiological.com/research/virus/respiratory-syncytial-virus
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.