Over the recent decade, significant advancements in chimeric antigen receptor T (CAR-T) cell therapy have occurred. As shown by an increasing number of successful clinical trials and FDA approval, CAR-T therapy has emerged to radically transform cancer treatment.
The FDA has approved six CAR-T cell therapies for treating blood cancers, including leukemia, lymphomas, and multiple myeloma. It has been reported that there were approximately 2,754 active cell therapy agents in the global immunotherapy pipeline in 2022. This is an increase of 36% from the same figure for 2021.
CAR-T has the greatest number of ongoing trials and pipelines of all immunotherapies, with a total of 857 trials and 1,432 pipelines globally, highlighting that it continues to be the most active and promising immunotherapy (see Figure 1).1
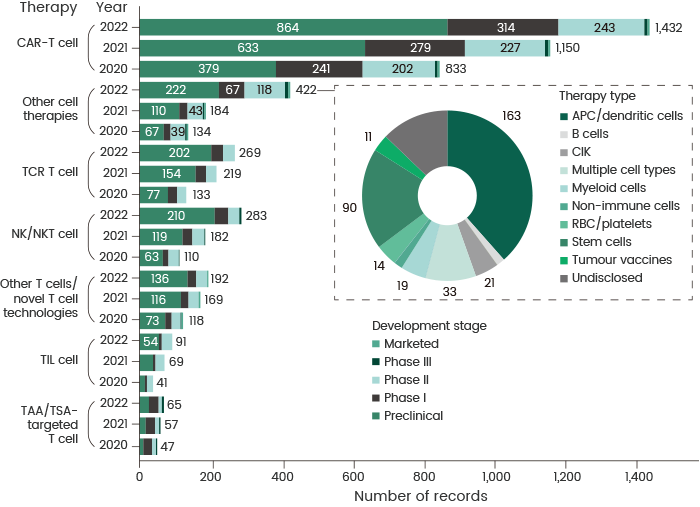
Figure 1. Changes in the cancer cell therapy pipeline by therapy type and year. Comparison of cell therapy agent development pipeline across various therapy types from 2020 to 2022. APC, antigen-presenting cell; CIK, cytokine-induced killer; NK, natural killer; RBC, red blood cell; TAA, tumor-associated antigen; TCR, T cell receptor; TIL, tumor-infiltrating lymphocyte; TSA, tumor-specific antigen. The pie chart shows the composition of the ‘other cell therapies’ category in 2022. Source: https://doi.org/10.1038/d41573-022-00095-1. Image Credit: Sino Biological Inc.
The successive production stages include collecting and expanding patients' T cells; CAR molecule design, screening, and transfection; target selection; and quality control (as displayed in Figure 2).
The main steps involved in CAR-T production are described below to offer insights into the difficulties that may hinder successful CAR-T therapy.
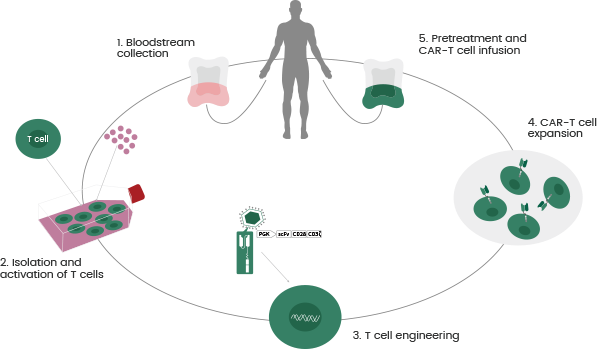
Figure 2. CAR T-cell Therapy Procedures. Image Credit: Sino Biological Inc.
CAR-T cell activation and expansion
CAR-T is one type of immunotherapy that utilizes a patient's own T cells for cancer treatment by exploiting the ability of the patient’s immune system to target and destroy cancer cells.
The initial step in CAR-T production is the collection of T cells from a patient. Since there is a limited number of cells, this necessitates expansion to a given amount (from billions up to tens of billions of CAR-T cells) before infusion back to the patient. This makes CAR-T cell expansion a vital step during development.
Cytokines stimulate the activation and expansion of CAR-T cells. For example, IL-7, IL-21, IL2, IL4, and IL15 are commonly utilized for CAR-T cell preparation and culturing.2
Due to the use of cytokines in CAR-T cell culturing, the grade or purity of cytokines and the concentration of remaining cytokines in the end product need strict control and monitoring to prevent any possible risks to the patient’s health.
As a result, the selection of high-quality cytokines and reliable cytokine detection methods, including cytokine ELISA kits, is growing in importance for CAR-T development.
CAR molecule design
The CAR molecule leads the CAR-T cells to the target on a given cancer cell’s surface and then activates them into cancer-killing agents. The CAR molecule is required to identify the cancer antigen precisely and specifically to enable cancer cells to be targeted while avoiding healthy cells.
To deliver improved recognition ability for CAR-T cells, the CAR molecule should be designed effectively since it impacts the persistence and durability of the CAR-T cells in the body.
A potent CAR molecule should hold several vital properties: high specificity for the target antigen, efficient CAR-T cell activation, and minimized off-target effects.
Extensive research efforts in recent years have focused on the CAR molecule optimization and have allowed its transition from a first-generation to a fourth-generation design (see Figure 3).3
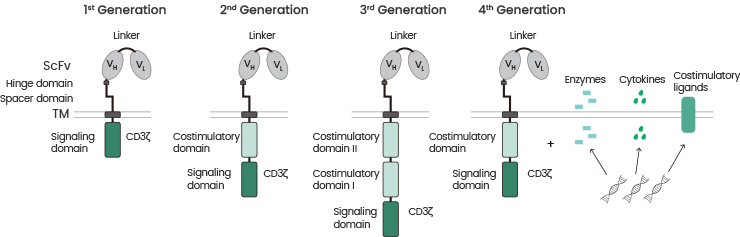
Figure 3. Chimeric antigen receptor generations. The 1st generation CART cells induced T cell activation only by the primary signal via the CD3ζ signaling domain. CART cells were further developed by integration of a costimulatory domain in 2nd generation CART cells. The 3rd generation CART cells consist of two costimulatory domains. The future 4th generation CART cells combine the vector with enzymes, cytokines, and costimulatory ligands. Source: doi:10.3390/ijms20246223. Image Credit: Sino Biological Inc.
The five common components of the CAR model are outlined below.
- Antigen-binding domain: This domain controls recognizing and binding to the target antigen on the cancer cell surface. This is usually an antibody fragment, such as a single-chain antibody fragment (scFv).
Efficient screening of the scFv molecule and conducting reliable affinity tests are the two most significant steps in developing a CAR molecule, affecting CAR-T therapy safety and efficacy.
- Costimulatory domains: These domains control the activation and expansion of CAR-T cells. Usually, they consist of signaling domains, such as 4-1BB or CD28, that deliver a secondary signal to the CAR-T cells to stimulate activation and expansion.
- Linker region: This region unites the costimulatory and antigen-binding domains. It regulates the transmission of signals between the two domains and is crucial for the correct functioning of the CAR molecule.
- Transmembrane domain: This domain links the intracellular and extracellular domains of the CAR-T cell, which secures the CAR molecule to the CAR-T cell surface. This domain is stable since it retains the proper orientation of the CAR molecule.
- Cytokine domains: Cytokines can improve CAR-T cell activation. For example, IL-2 can trigger cell proliferation and sustain cell viability during expansion.4
Some CAR molecules may also contain cytokine domains that secrete cytokines, stimulating the immune system and improving CAR-T cell activation and expansion.
CAR-T drug target selection and validation
As CAR-T is more capable of treating hematological malignancies than solid cancer, drug targets for hematological malignancies, such as BCMA, CD19, and CD22, continue to be the most popular target proteins, according to a 2022 report.1
Since two of the six FDA-approved CAR-T therapies utilize BCMA as a target and the remaining four utilize CD19, this suggests that these targets are significant and feasible.
Due to the competition among these popular targets, alternative novel targets, such as CLEC12A, GPRC5D, CD3, and CD7, have arisen in recent years. These alternatives show promise for revealing the potential for novel CAR-T discoveries.
In contrast to hematological malignancies, solid tumors have a more complex microenvironment, which restricts the capability of CAR-T cells to reach and penetrate tumors.
Drug targets for solid tumors need careful selection and screening. The most widely used drug targets for solid cancer include HER2, EGFR, PSCA, MUC1, MSLN, and PSMA.
During CAR-T production, drug targets are utilized for scFv screening and discovery in addition to CAR-positive rate detection. In CAR-T therapeutic products, target cells (CAR-positive T cells) that can correctly express both CAR and T-cell surface markers should be chosen to detect the proportion of target cells.
CAR-positive T cells are active components that are involved in tumor killing. The positive rate of CAR transfection is an essential test item for the product quality control of CAR-T cells.
Flow cytometry (FCM) persists as a broadly used technology in developing CAR-T cell therapies, particularly for identifying the phenotype and functionality of CAR-T cells.
As a result, the availability of high-quality reagents appropriate for FCM, such as numerous fluorescence-labeled drug targets and corresponding antibodies, is a key factor that could assist CAR-T development.
Challenges and future of CAR-T cell therapy
Due to several FDA approvals, CAR-T-cell therapy remains the most promising cell therapy for patients with hematological malignancies compared to alternative CAR-engineered cell therapies, including CAR-M and CAR-NK.
Some challenges need to be addressed to ensure the real success of CAR-T therapy in cancer treatment. Firstly, collection of T cells from patients may be time-consuming and complex, particularly for cancer patients with a compromised immune system and very few T cells.
Secondly, the use of CAR-T for the treatment of solid cancers is not as effective as for hematological cancer. This is due to the complicated and heterogeneous microenvironment of solid tumors, which inhibits CAR-T cells from reaching and infiltrating tumors.
Lastly, the availability and cost of CAR-T therapy restrict its utilization due to CAR-T cells (produced specifically from patients) being highly individualized, more expensive, and requiring a lengthier production period than alternative immunotherapy-based methods.
Researchers continue to actively explore various strategies to enable CAR-T therapy to be effective against solid tumors and more commercially viable.
Sino Biological is the leading supplier of bioreagents and CRO services for the biopharmaceutical field and offers comprehensive solutions for CAR-T cell therapy development. Their reagents and services assist clients through each stage, from early target discovery through to preclinical research and development.
References and further reading
- Landscape of cancer cell therapies: trends and real-world data. https://doi.org/10.1038/d41573-022-00095-1
- Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. https://doi.org/10.1182/blood-2014-01-552174
- The Application of CAR‑T Cells in Haematological Malignancies. doi.org/10.1007/s00005-020-00599-x
- The Role of Checkpoint Inhibitors and Cytokines in Adoptive Cell-Based Cancer Immunotherapy with Genetically Modified T Cells. doi: 10.1134/S0006297919070022
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.