Influenza – commonly known as the flu - is a family Orthomyxoviridae RNA virus that causes respiratory infection in mammals and birds. The virus is grouped into four main categories or types (A, B, C and D), which are characterized by variations in two main internal proteins: neuraminidase (NA) and hemagglutinin (HA).
Types A, B and C of the influenza viruses affect humans, and while Type D has not been known to infect humans, it is widely considered that infection is possible.
Type A can be found in a broad range of mammal and avian species, exhibiting significant changes in its immunological properties. In contrast, Type B is primarily restricted to humans and is regarded as a major cause of human morbidity.
Type C is not currently widely researched, but this is not felt to be an important cause of morbidity. Type D was only identified as recently as 2016.
Differences in the membrane proteins - neuraminidase (NA) and hemagglutinin (HA) - have resulted in Type A being divided into subtypes depending on the foremost targets for the body’s immune system.
The notation HhNn is utilized to describe the subtype consisting of the hth discovered hemagglutinin and nth discovered neuraminidase proteins.
The influenza viral hemagglutinin protein is a homotrimer with a receptor binding pocket on the globular head of each monomer, and the influenza viral neuraminidase protein is a tetramer with an enzyme active site on the head of each monomer.
Subtypes are additionally split into strains, with each genetically distinct virus isolate generally regarded as a distinct strain.
Influenza virus genome
The influenza virus genome is comprised of segmented negative-sense single-strand RNA. Influenza viruses are members of the family Orthomyxoviridae, which contains four genera: Types A, B, C and Thogotovirus. Genera A and B are the only types regarded as clinically relevant to humans.
Each of the eight genome segments of the influenza Type A and Type B viruses are loosely encapsidated by the nucleoprotein (NP). The influenza genome is comprised of eight genes encoding eleven proteins.
The polymerase complexes are comprised of the three polymerase proteins - PB1, PB2 and PA - which are located at the ends of the nucleocapsids.
The helical capsids are enclosed by the M1 matrix protein and a host-derived lipid bilayer envelope. The M2 matrix protein and the virus surface glycoproteins haemagglutinin and neuraminidase are embedded.
Haemagglutinin is synthesized as a precursor protein and cleaved via cellular serine proteases into functional proteins: HA1 and HA2, plus proteins NS1 (nonstructural protein 1) and NS2 (nuclear export protein or NEP). The specific roles of these are still being explored.
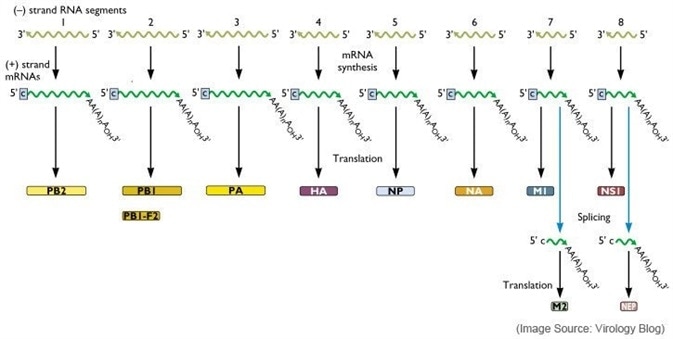
Image Credit: Sino Biological Inc.
Influenza virus types and classifications
Influenza viruses are RNA viruses comprising four of the seven genera of the family Orthomyxoviridae, identified as Types A, B, C and Influenza D.
Influenza Type A viruses may infect humans, pigs, birds and other wildlife, spreading the virus globally. It is believed that human Type A influenza viruses are derived from strains that originally infected wild aquatic birds.
Influenza B viruses infect humans and seals, and these are known to mainly affect children. Influenza C viruses generally result in mild or subclinical infections in patients infected with the virus.
While influenza Types A and B lead to seasonal epidemics, only influenza Type A has caused worldwide pandemics, accounting for approximately two-thirds of human infections annually.
Table 1. Classification / types of Influenza virus. Source: Sino Biological Inc.
| |
Influenza A |
Influenza B |
Influenza C |
| Hosts |
Humans, waterfowl, poultry, pigs, horses, sea mammals, bats |
Humans, seals |
Humans, pigs, dogs |
| Gene segments |
8 |
8 |
7 |
| Proteins |
11 |
11 |
9 |
| HA/NA antigenic subtypes |
18 HA, 11 NA |
None |
None |
| Clinical features |
Moderate to severe illness |
Milder disease than Influenza A |
Largely subclinical |
| Epidemiological features |
Causes pandemics |
Less severe epidemics than Influenza A; no pandemics |
Does not cause epidemics or pandemics |
Influenza virus structure
Influenza viruses possess segmented genomes. Influenza Type A viruses (IAVs) and Type B viruses (IBVs) contain eight negative-sense, single-stranded viral RNA (vRNA) gene segments. These encode transcripts for ten crucial viral proteins, plus a number of strain-dependent accessory proteins.
Influenza Type C and D viruses, on the other hand, possess only seven vRNA gene segments. The hemagglutinin-esterase fusion protein vRNA replaces the neuraminidase (NA or N) and hemagglutinin (HA or H) vRNAs.
Influenza Type A is the main pathogen in seasonal influenza and the cause of influenza pandemics. The influenza Type A genome encodes eleven viral proteins:
- Hemagglutinin (HA), which is divided into two subunits (HA1 and HA2)
- Two matrix proteins (M1 and M2)
- Neuraminidase (NA)
- Nucleoprotein (NP)
- Heterotrimeric RNA-dependent RNA polymerase, comprising three subunits:
- One polymerase acidic (PA)
- Two polymerase basic (PB1 and PB2)
- The alternatively transcribed proapoptotic peptide (PB1-F2)
- Two nonstructural proteins (NS1 and NS2). NS2 is also known as NEP or nuclear export protein.
The virus particle (virion) has an irregular spherical shape with a lipid envelope. This has a diameter of approximately 80-120 nm.
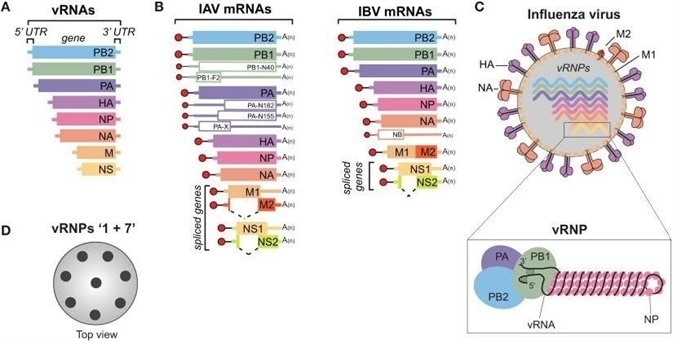
Image Credit: Sino Biological Inc.
Influenza virus replication
The influenza virus replicates itself within the nucleus of a host cell, as can be seen in the virus replication cycle below:
- Cell Binding and Fusion: HA bind with SA residue
- Genome Trafficking to the Host Cell Nucleus: With vRNP trafficking to the nucleus, NP, PA, PB1 and PB2 are the viral proteins that make up the vRNP.
- Transcription and replication of the viral genome: Replication of the influenza genome comprises two steps: Transcription of complementary RNA (cRNA) and transcription of new vRNA copies utilizing the cRNAs as templates.
- Assembly and Trafficking of vRNPs
- Export of vRNPs from the nucleus: vRNPs appear to be exported out of the nucleus via the CRM1 dependent pathway via the nuclear pores.
- Assembly and budding at the host cell's plasma membrane: Virus particles will bud from the apical side of polarized cells.
Influenza virus symptoms
A characteristic clinical indicator of human influenza virus symptoms is a sudden rise in body temperature to >38.5°C. This generally occurs between one and three days after infection. Other symptoms include:
- Fever
- Limb ache
- Headache
- Lethargy
- Dry cough
- General faintness
- Encephalitis
- Myocarditis
Influenza can lead to complications, including metabolic disorders and chronic heart or lung disease.
Infectivity can occur shortly (<24 hours) before the onset of clinical symptoms and usually persists for a period of three to five days. Small children can excrete the viruses earlier and over a longer period than adults, while the most serious outcomes include:
- Peracute death within a matter of hours
- Primary influenza pneumonia
- Myocarditis
- Encephalitis
Influenza virus treatment
Two classes of antiviral drugs are authorized for the treatment of influenza: neuraminidase inhibitors such as oseltamivir, laninamivir, zanamivir and peramivir and M2 protein inhibitors - derivatives of adamantane.
Also, vaccination is the most effective way to prevent flu infection. Each year, several different flu strains are selected as vaccine strains based on surveillance data of the recent isolates, and the performance of the vaccines from the previous season.
Sino Biological offers recombinant Flu antigen products under its ProVirTM viral antigen collection. The product line covers Hemagglutinin (HA), Neuraminidase (NA), and Nucleoprotein (NP) proteins, from all from all WHO-recommended vaccine strains in recent years. These antigens can be used to analyze vaccine-induced antibody response. In addition, Sino Biological also develops a large collection of monoclonal antibodies against the flu antigens. These reagents can help facilitate relevant assay development.
References
- Arbeitskreis Blut.Influenza Virus.Transfus Med Hemother. 2009 Feb; 36(1): 32–39.
- Dan Dou. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement.Front Immunol. 2018; 9: 1581.
- Tasleem Samji.Influenza A: Understanding the Viral Life Cycle.Yale J Biol Med. 2009 Dec; 82(4): 153–159.
- Scott H. James, Richard J. Whitley, in Infectious Diseases (Fourth Edition), 2017
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. Sino Biological has independently developed 6000 recombiant proteins, 85% of which are expressed in eukaryotic cells making them closer to natural structure. Sino Biological is dedicated to virology and infectious disease research. The ProVirTM collection is the world’s largest viral antigen bank, carrying over 800 products from 350 strains of viruses. Sino Biological is the first company in the world to produce and supply the SARS-CoV-2 viral protein reagents.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.