Mar 18 2015
An international team of researchers from the CEA, the CNRS, the Institut Laue-Langevin, the Jülich Centre for Neutron Science, the University of California Irvine, the Australian Institute of Science and Technology Organisation, the Max Planck Institute Mülheim and the University of Perugia has shed light on the molecular mechanism behind the importance of water for functional protein dynamics. The scientists have discovered that water’s ability to flow on the surface of proteins makes them sufficiently dynamic to be biologically active. The results have just been published in Nature Communication on 16/03/2015.
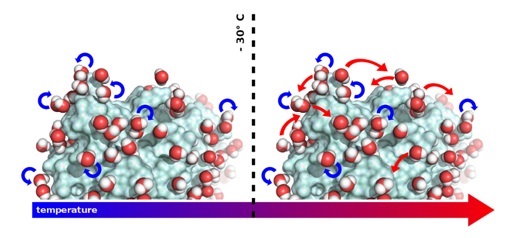
In order to be biologically active, most soluble proteins require their surface to be covered with water. This so-called hydration water is generally acknowledged to enable a protein to undergo the internal motions that are so fundamental for its capacity to fulfill a specific biological function. Yet, the molecular mechanism behind water’s importance for functional protein dynamics has remained elusive. The team has now been able to observe the movements of water molecules on the surface of proteins. The study highlights how these movements correlate with protein dynamics that is essential to biological activity. Temperature turned out to be an essential parameter; since the motions of water molecules and, therefore, the behaviour of the proteins depend on it.
When "visualising" water movement on the protein surface, the team discovered that the molecules rotate around their own axes at temperatures below ‑30°C, temperatures at which proteins are inactive. Above ‑30°C however, whilst continuing to rotate, the water molecules also start to undergo translational diffusion. This is the temperature at which proteins start to be active and the researchers suggest that the capacity of water molecules to "dance" on the surface of proteins enables the dynamics they need to function.
To achieve these results, the scientists combined neutron scattering with molecular dynamics simulations. The neutron scattering technique provides detailed information on the movement and local arrangement of atoms and molecules in matter. The researchers had to first mask the scattering signal of the proteins, whilst preserving the signal from the water molecules on the protein surfaces. To achieve this they produced perdeuterated proteins (proteins in which all the hydrogen atoms are replaced by deuterium atoms) in ILL’s Life Sciences Group.
The study provides a better understanding of the conditions proteins require to be biologically active. An application of the results is the stabilization of protein drugs in the solid state, such as for example insulin that is used for treating diabetes.